India’s Central Board of Indirect Taxes and Customs published Circular No.15/2023-Customs in which mandatory additional qualifiers in import/export declarations shall be made such as scientific names, IUPAC names, brand names as applicable, to aid in reducing queries and improve the efficiency of assessment. The Shipping Bill (Electronic Integrated Declaration and Paperless Processing) Regulations 2019 was scheduled to be implemented on July 1, 2023, and has been postponed until October 1, 2023, because of strong opposition from the chemical industry.
The following are chemicals involved:
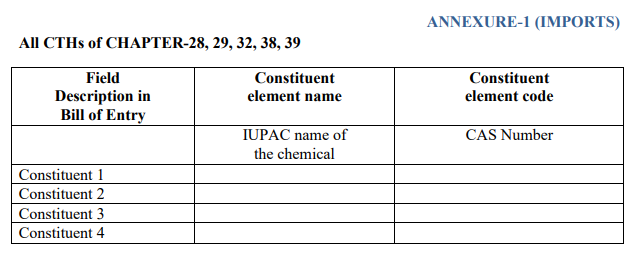
Imports : Imported products prescribed under chapters 28, 29, 32, 38, and 39 of the Customs Tariff Act, 1975, (including inorganic chemicals; precious metals; rare earth metals; organic or inorganic compounds of radioactive elements or isotopes; organic chemicals; tanned or dyed extracts; tannins and their derivatives; dyes, pigments, and other colorants; paints and varnishes; putty and other adhesives; ink; miscellaneous chemical products; plastics and their products). The International Union of Pure and Applied Chemistry (IUPAC) name and CAS number of chemicals must be provided in the declaration.
Exports :
a. the declaration of the name of the medicinal plant, for exports of parts of plants under chapter 12;
b. the declaration of the name of the formulation, for exports of formulations of different streams of medicine under chapter 30;
c. the declaration of the surface material that comes into contact with the chemical, for exports of various products under chapter 84.
An example of the declaration form on additional information:
CIRS Comments
Once implemented, this Regulation will have a significant impact on the protection of Confidential Business Information (CBI) for companies, as it will prevent the confidentiality of critical components. Currently, various associations and groups from numerous countries have already begun expressing their opinions on this Regulation, focusing on excessive disclosure of information. As such the deadline has been postponed but it is recommended that companies, regulatory authorities, and industry associations submit their opinions. Additionally, it remains unclear whether third parties will be allowed to submit relevant information.
If you need any assistance or have any questions, please get in touch with us via service@hfoushi.com.
Further Information

