On November 23, 2022, South Korean Ministry of Environment (MoE) released 11 candidate substances subject to authorization for public consultation in its Announcement No. 2022-671. Comments can be made from December 15, 2022 to February 13, 2023 (lasting 60 days).
Substances subject to authorization under K-REACH are required to obtain the authorization from MoE prior to production, import or use in South Korea, even if the substances have already completed registrations under K-REACH.
The following are 11 candidate substances:
|
Serial Number |
Substance Name |
CAS No. |
|
1 |
Benzene |
71-43-2 |
|
2 |
Bisphenol A (BPA) |
80-05-7 |
|
3 |
Dibutyl phthalate |
84-74-2 |
|
4 |
Benzyl butyl phthalate |
85-68-7 |
|
5 |
4,4'-methylenebis[2-chloroaniline] |
101-14-4 |
|
6 |
Bis(2-ethylhexyl) phthalate |
117-81-7 |
|
7 |
Orange lead |
1314-41-6 |
|
8 |
Lead monoxide |
1317-36-8 |
|
9 |
Chromium trioxide |
1333-82-0 |
|
10 |
Lead sulfochromate yellow |
1344-37-2 |
|
11 |
Strontium chromate |
7789-06-2 |
According to the plan, Korean MoE will publish information of the 11 substances including their hazards, circulation amount and specific uses on December 12, 2022 and collect public comments from December 15, 2022 to February 13, 2023 (lasting 60 days).
Any opinions on these 11 candidate substances can be submit via the link below: www.chemnavi.or.kr.
Opinions shall cover three aspects, including:
- Basic information of stakeholders;
- Information on institutions and organizations to which stakeholders belong (“Non-disclosure” is available);
- Opinions can be made as following (Either “non-disclosure” or “partial non-disclosure” is available):
- If there is any disagreement regarding the hazards of the substance, a statement should be made;
- If there is any disagreement regarding the main uses or there are additional uses of the substance, a statement should be made;
- If there is any disagreement regarding the exposure information or there is additional exposure information of the substance, separate statements should be made for different uses and exposure routes;
- Limitations on alternative substances and technologies in current R&D applications should be stated;
- Opinions on potential exposures to relevant personnel should be made during the processing of designated substances;
- Potential socio-economic influences should be stated after substances are designated to be subject to authorization;
- Opinions on the circulation amount of 11 substances in Korea should be made;
- Opinions wishing to be exempted from authorization for specific uses and to obtain a grace period for authorization should be stated.
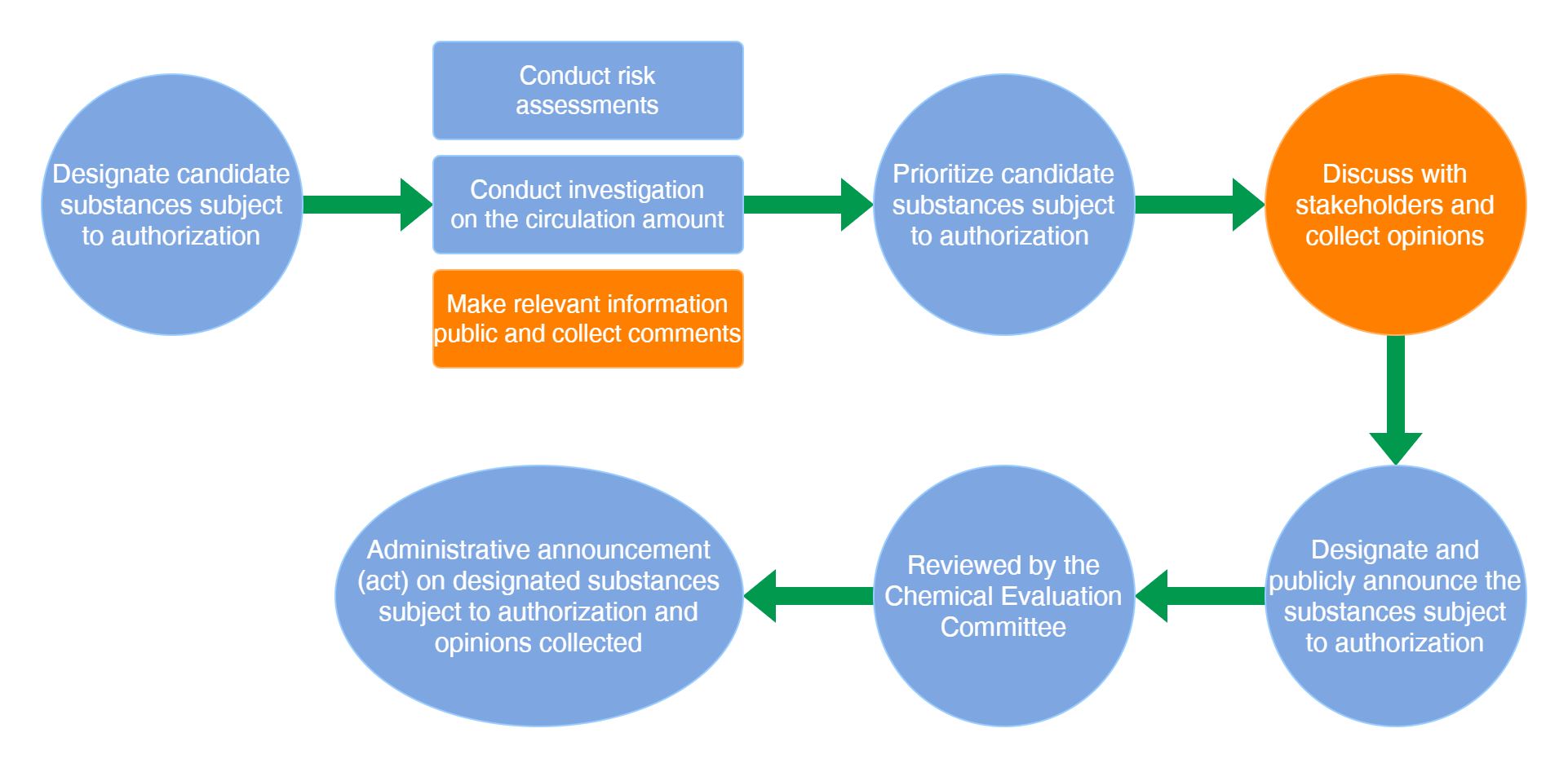
The main procedures for designating substances subject to authorization are as below:
If you need any assistance or have any questions, please get in touch with us via service@hfoushi.com.

