The master files system is to solve the contradiction between the enterprise's need to protect trade secrets and the NMPA reviewer's requirement for full disclosure of information. Years of operating experience of the system on a global scale have proved its significance in the review and approval process of drugs and medical devices.
Regulation:
Announcement of the NMPA on Further Improving the Joint Review, Approval, and Supervision of Drugs (No. 56 of 2019)
Scope of application:
Applicable to medical device registration applicants for the registration, registration change, and clinical trial approval of imported Class II, Class III and domestic Class III medical devices (including in vitro diagnostic reagents).
Who can register?
Owner of the master files: A manufacturer that expects or has provided raw materials or intermediates for medical device applicants.
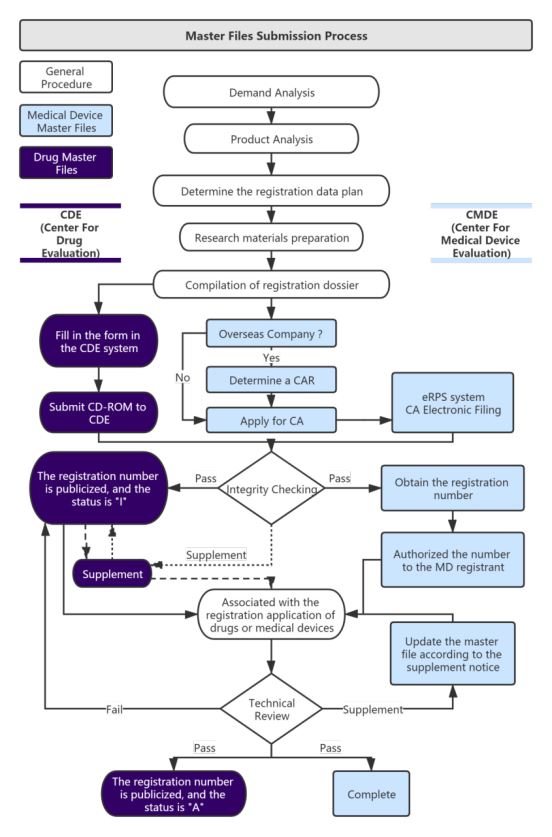
Service process:
Our services:
Medical device raw materials master files submission
Medical device raw materials master files change
Pharmaceutical excipients submission
Pharmaceutical packaging materials submission