1.Medical Device Registration System
Regulation system:
Regulations on the Supervision and Administration of Customized Medical Devices (Trial) (Announcement No. 53 of 2019)
57 guidelines for technical review of medical devices, including the "Guidelines for Medical Devices' Conditional Approval to Market".
Reform of review and approval system:
"Announcement on Adjusting the Approval Procedure for Clinical Trials of Medical Devices" (Announcement No. 26 of 2019).
Announcement on the Publication of New and Revised Catalogues of Medical Devices Exempted from Clinical Trials (Announcement 91 of 2019)
The "Notice of the NMPA on Expanding the Pilot Work of the Medical Device Registrant System" extended the pilot of the medical device MAH system to 21 provinces, autonomous regions, and municipalities including Beijing, Jiangsu, and Zhejiang. In 2019, a total of 93 products from 22 companies were approved in accordance with the MAH system.
2.Application for registration of medical devices
In 2019, the NMPA accepted a total of 9,104 applications for the first registration, renewal of registration, and Alteration for medical devices. Compared with 2018, the number of registration acceptance projects increased by 37.8%.
3,511 applications for registration of Class III medical devices in China, and 5,553 applications for registration of imported medical devices. Compared with 2018, it has increased by 47.4%. Among them, there were 2,154 applications for medical device registration and 1,357 applications for in vitro diagnostic reagent registration.
According to the registered varieties, there are 5877 applications for medical device registration and 3227 applications for in vitro diagnostic reagent registration. Registration form is shown in Figure 1。
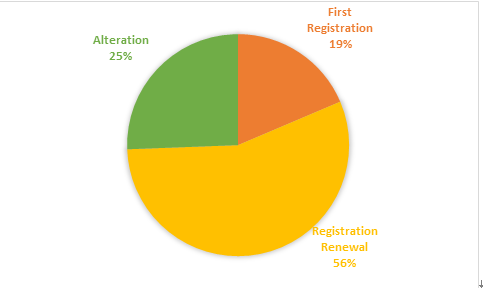
Figure 1
There were a total of 3053 registrations for the import of Class II medical devices, an increase of 43.7% compared with 2018. Among them, there are 1,559 medical device registration applications and 1,494 in vitro diagnostic reagent registration applications.
A total of 2,540 registrations for imported Class III medical devices were accepted, an increase of 20.9% compared to 2018. Among them, there were 2164 medical device registration applications and 376 in vitro diagnostic reagent registration applications.
3.Examination and approval of medical device registration
In 2019, the NMPA approved a total of 8,471 first registrations, renewal registrations, and alteration registrations of medical devices, compared with 2018, the total number of registration approvals increased by 53.2%. Among them, 1,726 were registered for the first time, 4,504 were renewed, and 2,241 were changed in terms of permits.
The registration of medical devices approved by the NMPA in the past 6 years is shown in Figure 2.
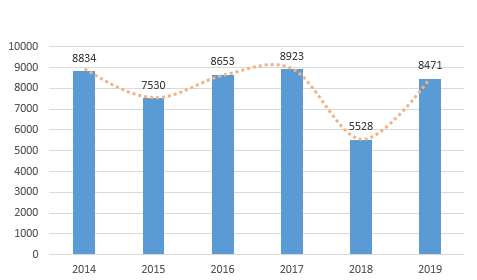
In 2019, the NMPA approved 3179 domestic Class III medical device registrations, an increase of 86.0% compared with 2018, and 5,292 imported medical devices, an increase of 38.6% compared to 2018. According to the registered varieties, there were 5,226 medical devices, accounting for 61.7% of the total registered medical devices; 3245 in vitro diagnostic reagents, accounting for 38.3% of the total registered medical devices.
There were 2754 registrations of imported Class II medical devices. Among them, there are 1,521 medical device registrations and 1,233 in vitro diagnostic reagent registrations.
There were 2,538 registrations of imported Class III medical devices. Among them, there were 2,206 medical device registrations and 332 in vitro diagnostic reagent registrations.
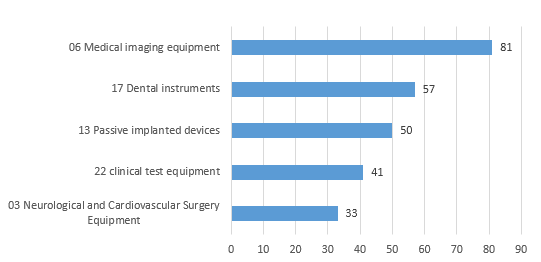
Except for in vitro diagnostic reagents, the registered imported medical devices involve products in 23 sub-categories of the Medical Device Classification Catalog.
The top five imported medical devices in the registered quantity are mainly medical imaging devices, stomatological devices, passive implant devices, clinical inspection devices, neurological and cardiovascular surgical devices.
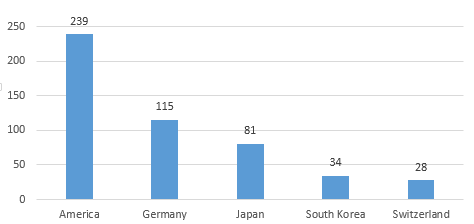
In 2019, the United States, Germany, Japan, South Korea, and Switzerland ranked the top 5 for the first registration of medical device imports in China. The number of registered products accounted for 75.4% of the total number of first registration of imported products in 2019, which was basically the same as in 2018.
4.The registration and approval of innovative medical devices and other products
In 2019, the NMPA received a total of 179 applications for special approval for innovative medical devices, 36 of which were approved for special review; 31 applications for priority approval were received, 12 were approved for priority approval, and 19 innovative medical device products were approved.
Source: https://www.cmde.org.cn/CL0004/20623.html

