In order to further strengthen the management of clinical trials of medical devices, safeguard the rights and interests of the subjects in the clinical trials of medical devices, promote the transformation of scientific research results, improve the efficiency of approval, and accelerate the listing of products, the NMPA revised theClass III Medical Devices that Need to Be Approved for Clinical Trials" and drafted theClass III Medical Devices that Need to Be Approved for Clinical Trials (Revised in 2020, draft for comments).
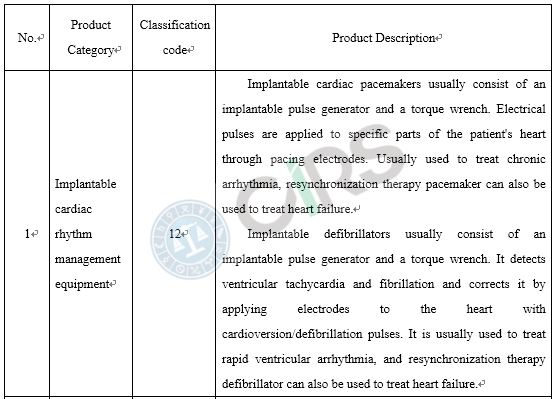
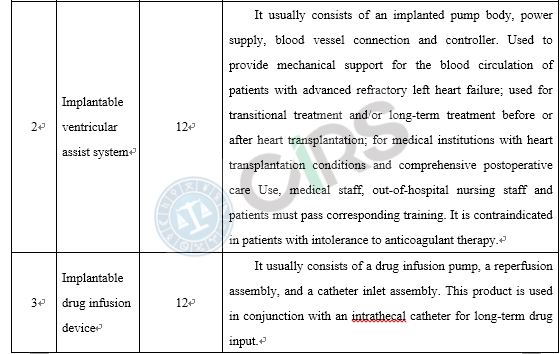
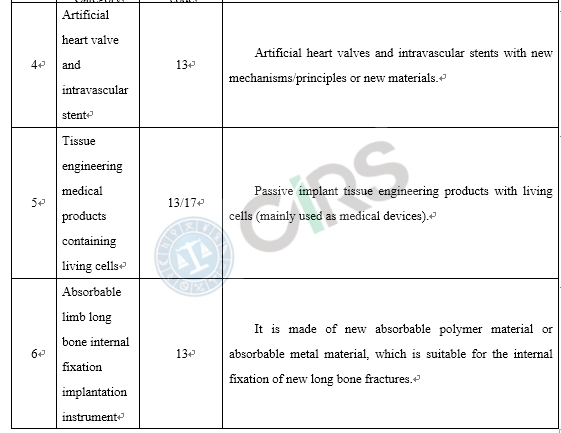
Compared with the products already on the market, medical device products adopt new designs, materials or mechanisms, and/or are applicable to a new scope of application, requires clinical trials in China. In order to protect the rights and interests of clinical trial subjects, those medical devices that require clinical trials in China and have a higher risk to humans should be subject to clinical trial approval. These include the following scenarios: