Under the background of the promotion of CBEC policies, the improvement of logistics networks, and the vigorous development of major cross-border platforms, CBEC has become an important channel for overseas health food sales.
Different from traditional trade which requires obtaining the registration or filing certificate prior to import into China, health food entering the Chinese market through CBEC retail does not require those certificates at present. Therefore, in recent years, many overseas health food, particularly the health food of new brands, have entered the Chinese market by CBEC and some of them have gradually accumulated great reputation and popularity among consumers in China.
What is CBEC?
Definition
CBEC mostly refers to CBEC retail.
- CBEC retail: It refers to a transaction between trading entities that belong to different Custom Territories. In order to achieve transactions and payment, the transaction will be completed through the computer networks. And the commodities will be delivered to consumers through cross-border logistics such as express mail, parcels and other postal methods. It generally refers to B2C (business-to-customer) mode which is for individuals.
Classification
CBEC ( B2C) can be divided into two modes of operation:
- Bonded Import: stocking in bonded warehouse – orders from consumers – sending products from bonded warehouse.
-
Direct Purchase Import:
orders from consumers - sending products from overseas.
Changes in CBEC Policy
Stage I: Since 8 April 2016, the new tax on CBEC (B2C) includes customs duties, value added tax (VAT), and consumption tax. Only the commodities listed in Positive List can be imported to China through CBEC (B2C). Since 1 July 2016, the health food for the first importation needs to get registration or filing approval before importing through CBEC (B2C).
Stage II: Since 24 May 2016, government provided one year transition period for the policy of the Positive List, health food registration or filing is not required for CBEC (B2C) temporarily. The deadline is 11 May 2017. The transition period was later extended to 31st December, 2017.
Stage III: Since 1 January 2018, CBEC (B2C) import commodities have been temporarily supervised as personal items. The new supervision is carried out in 15 comprehensive experimental areas. The transition period was later extended to the end of 2018. Therefore, before 31st December, 2018, health food registration or filing is not required for CBEC (B2C) import commodities.
Health Food Importation Process through CBEC (Take Bonded Import as an Example)
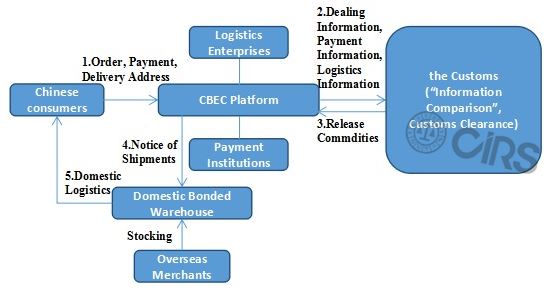
In order to assist overseas health food companies in CBEC retail, more detailed information on CBEC will be introduced in
Section 6 of
- Introduction of CBEC
- The Development of CBEC Policy
- Health Food Importation Process through CBEC
- Compliance Procedures of Health Food Importation through CBEC
The summary of each section in the Guideline is introduced by a series of articles which are available as following. If it is of your interest, please kindly click to get information.
Section 1: Information to Know before Health Food Exportation to China
Section 2: How Many Health Food are Registered in China?
Section 3: Chinese Health Food Regulation in Present and Future
Section 4: Timeline and Budget for Health Food Access to Chinese Market
Section 5: How does Chinese Government Supervise the Health Food?
Section 6: Cross-Border E-Commerce (CBEC): New Opportunity for Imported Health Food to Enter the Chinese Market
In addition, if you would like to get the
Editor:
CIRS Food Technical Team: Established in 2012, CIRS Food Technical Team has more than 80% masters with degrees of Food Safety or Food Engineering and more than 50% members from oversea renowned universities. Since it was founded, based on the rich experience of regulatory compliance and deep understanding of Chinese food regulations and industry, food specialists have made comprehensive and reasonable solutions for many oversea food companies to complete Chinese food regulatory compliance progress.
If you have any other questions, please contact us at service@hfoushi.com.
Related Article
Cross-Border Acquisition: Chinese-funded Enterprises Frequently Acquire Foreign Health Food Brand
Data Analysis: Why are Health Food Enterprises Keen to Explore E-Commerce Channel?

