In order to help enterprises better understand the status of health food (dietary supplements) filing in China, CIRS Group has gathered statistics on health foods approved in 2023 and analyzed them from multiple perspectives.
Related links
China Health Food Registration and Filing
Analysis on the Filing Status of Health Food (Dietary Supplement) in 2022 in China
Analysis on Health Food (Dietary Supplement) Registration in 2023 in China
The Filing Status of Health Food in China
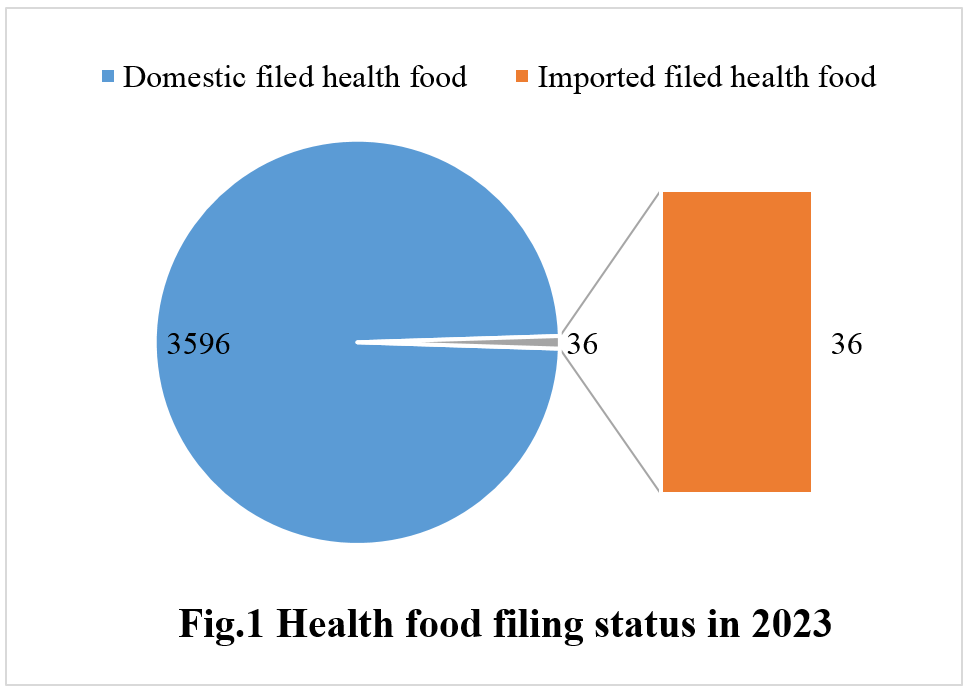
As of December 31, 2023, according to the information released by the Special Food Information Query Platform of the State Administration for Market Regulation (SAMR), a total of 3,632 health foods obtained filing certificates, of which 3,596 are domestic and 36 are imported.
The Filing Status of Health Food in Different Regions
Domestic health food
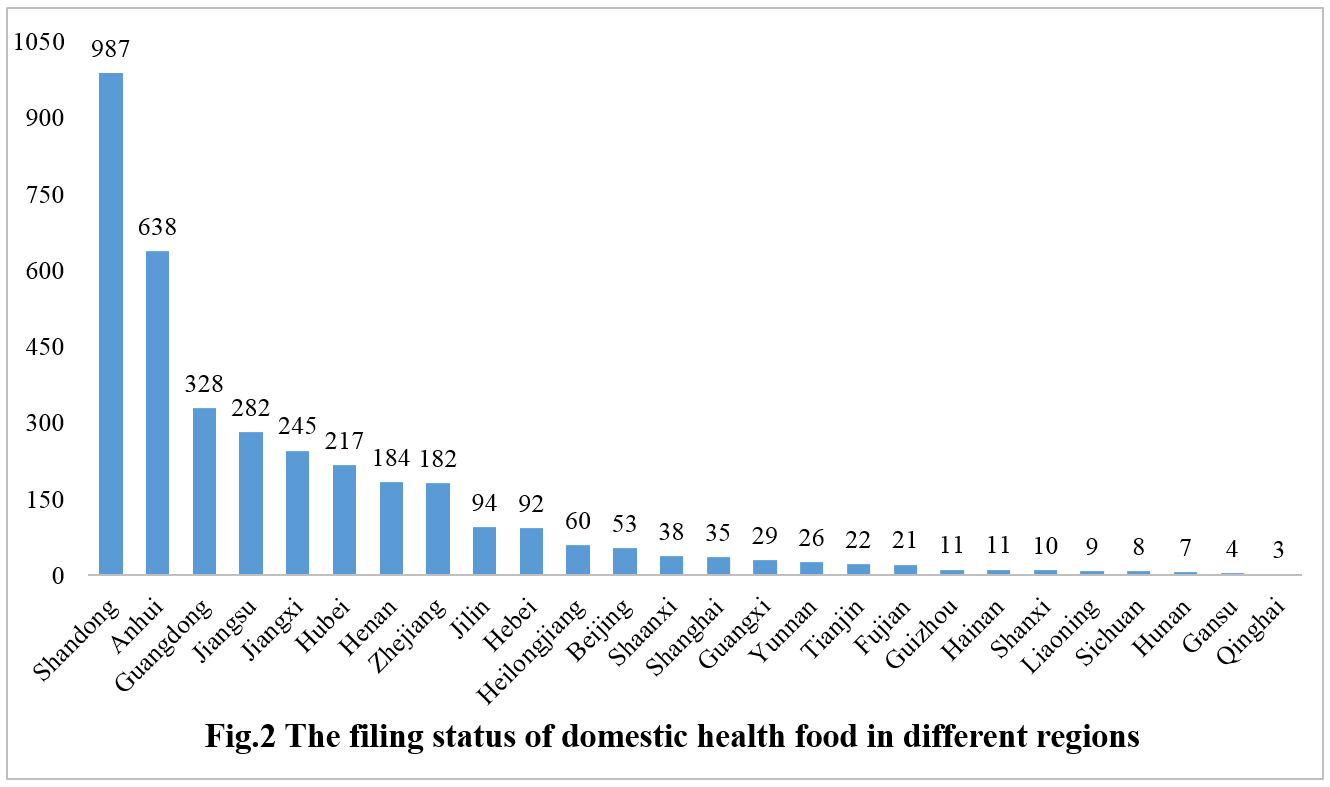
The filing status of domestic health foods varies significantly across different regions in China, including provinces, directly governed municipalities, and autonomous regions. Shandong province obtained a total of 987 health food filing certificates, securing the top spot in terms of the number of filings. Anhui province and Guangdong province secured the second and third positions with 638 and 328 certificates, respectively.
Imported health food
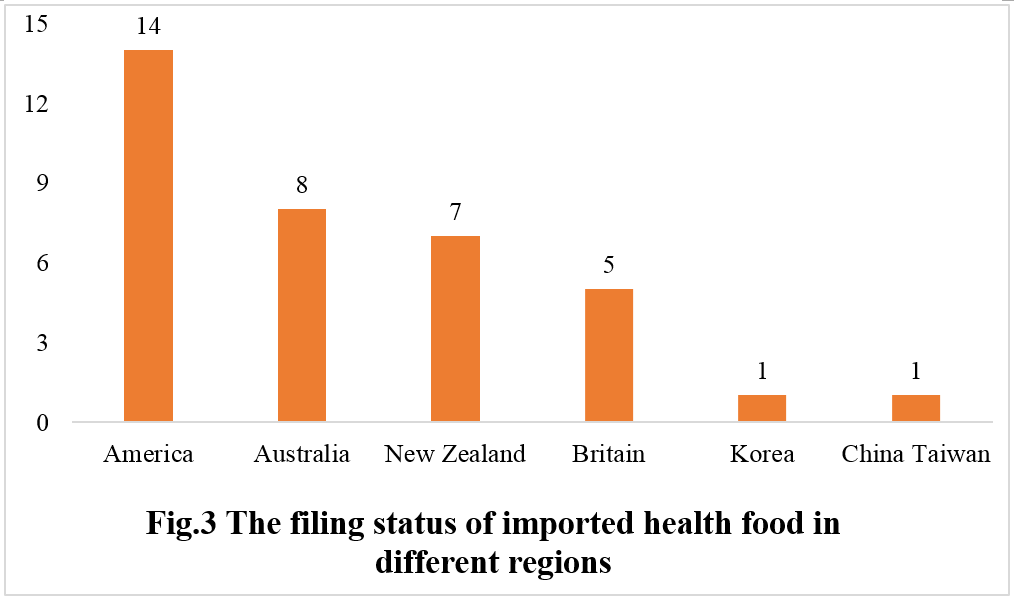
Applicants from the United States, Australia, New Zealand, Britain, Korea, and China's Taiwan have obtained the imported health food filing certificates. Among the 36 imported filing products, 14 are from U.S. companies, 8 from Australian companies, and 7 from New Zealand companies.
The Filing Status of Health Food in Different Enterprises
Domestic health food
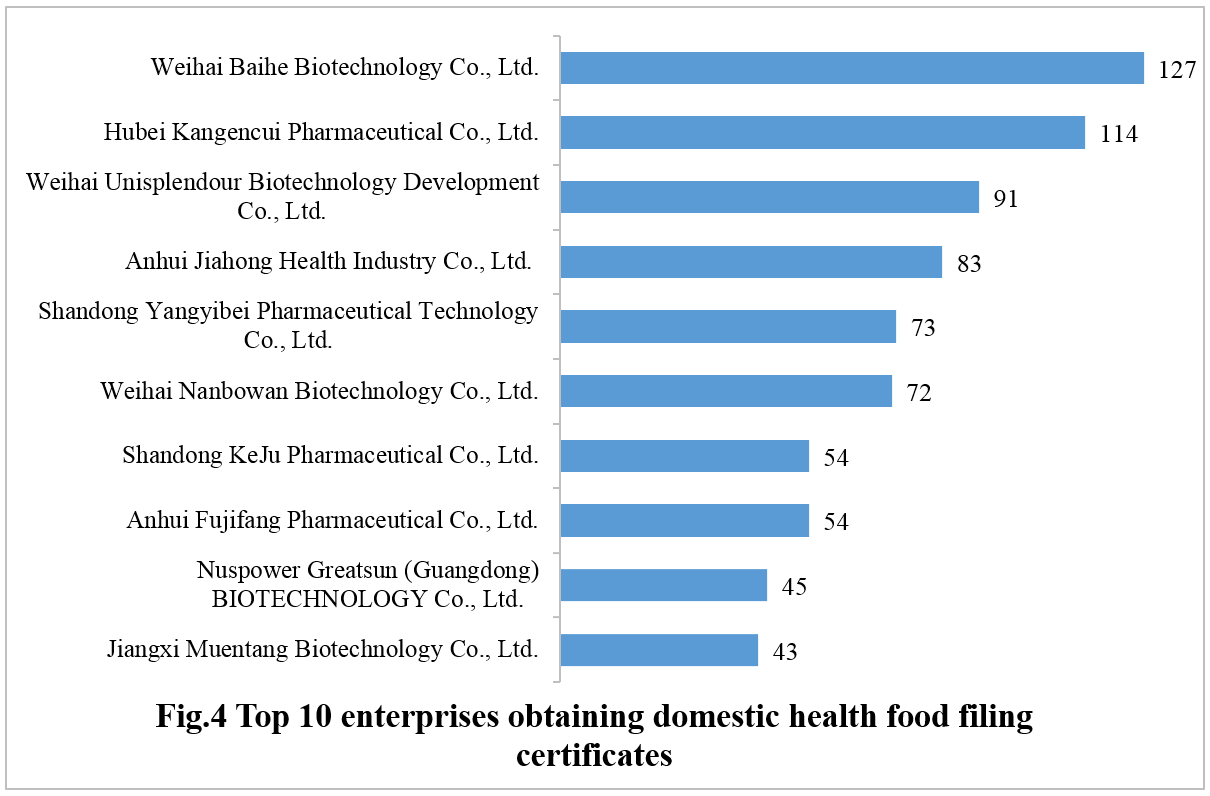
576 domestic health food enterprises have obtained the filing certificates. Weihai Baihe Biotechnology Co., Ltd. has the largest number of approvals with a total of 127 filed products, followed by Hubei Kangencui Pharmaceutical Co., Ltd. and Weihai Unisplendour Biotechnology Development Co., Ltd., with 114 and 91, respectively.
Imported health food
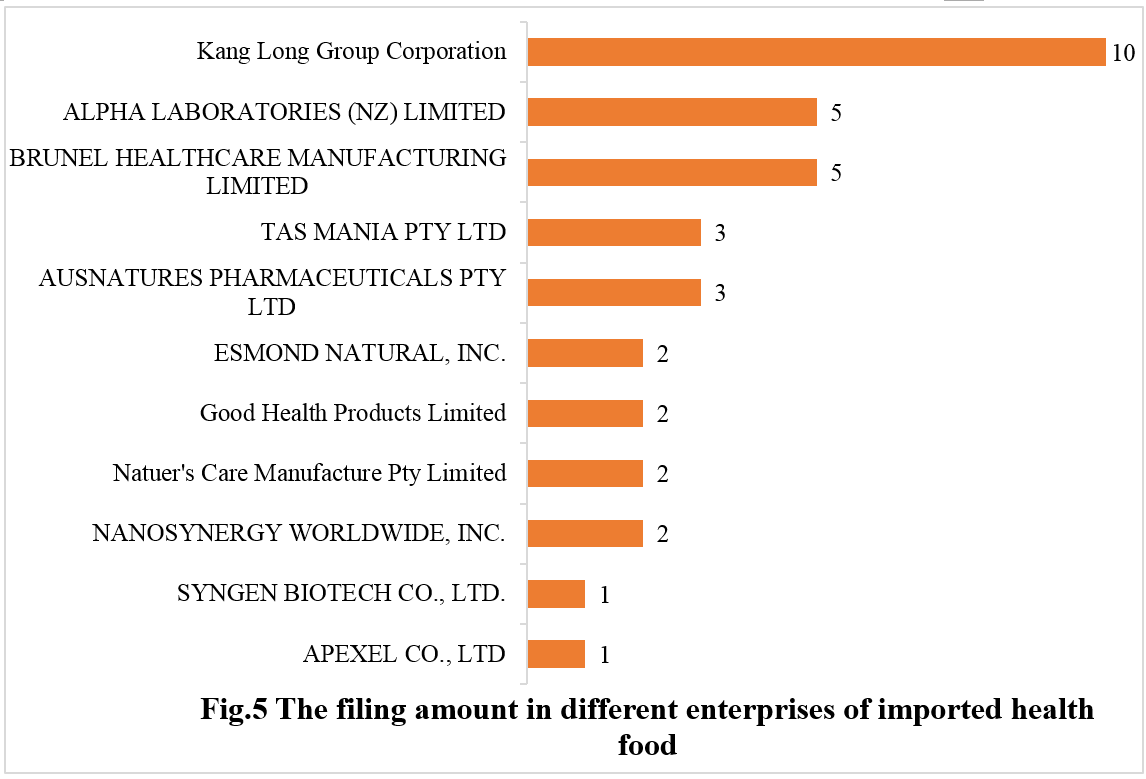
11 imported health food enterprises have obtained the filing certificates. Kang Long Group Corporation from the United States claimed the top spot with 10 filed products, followed by ALPHA LABORATORIES (NZ) LIMITED from New Zealand and BRUNEL HEALTHCARE MANUFACTURING LIMITED from Britain, with 5 each for both.
The Filing Status of Health Food in Different Dosage Forms
At present, the permitted dosage forms for filing include tablets, capsules (hard/soft), oral liquids, granules, powders, and gelatin candy (gummies).
Domestic health food
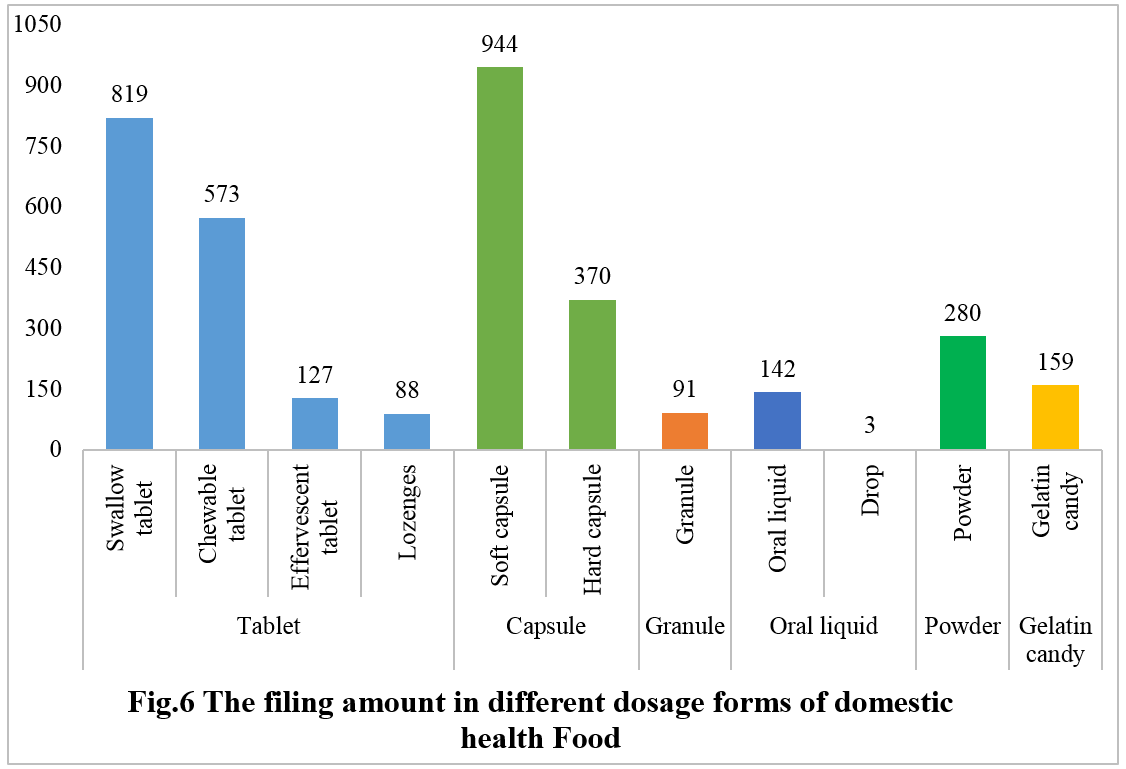
In 2023, the filed domestic health foods were primarily in tablet form, totaling 1,607, accounting for 44.7% of the total domestic health food filing throughout the year. Capsule products had a total of 1,314 filings, covering 944 for soft capsules and 370 for hard capsules. Additionally, there were 145 for oral liquids (including drops) and 91 for granules, with 3 specifically for drops. Powdered and gelatin candy products had 280 and 159 filings, respectively.
Imported health food
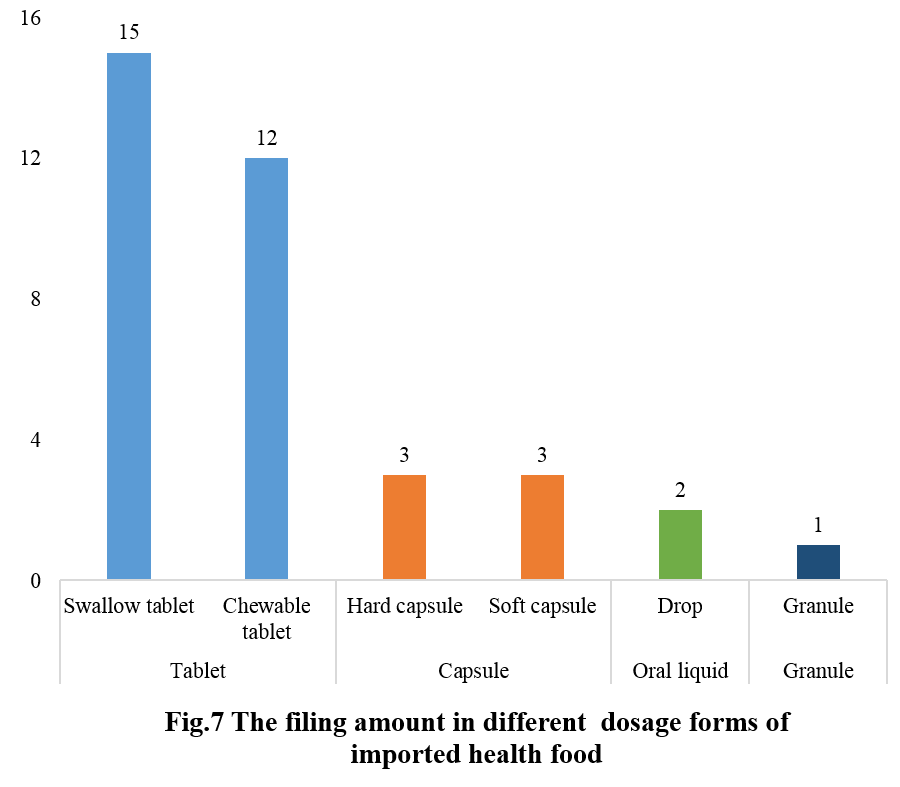
The filed domestic health foods in 2023 were predominantly in tablet form, with a total of 27 filings. Among them, there were 15 for oral tablets and 12 for chewable tablets. Capsule products had 6 filings, with 3 each for hard capsules and soft capsules. There were 2 filings for oral liquid products, both in drop form, and 1 for granules.
The Filing Status of Health Food with Different Functional Components
Domestic health food
Among the filed domestic health foods in 2023, there are 2,296 nutrition supplements, and 1,300 functional health foods with broken ganoderma lucidum spore powder, coenzyme Q10, melatonin, fish oil, or spirulina as the single raw material, accounting for 63.8% and 36.2% of the total, respectively.
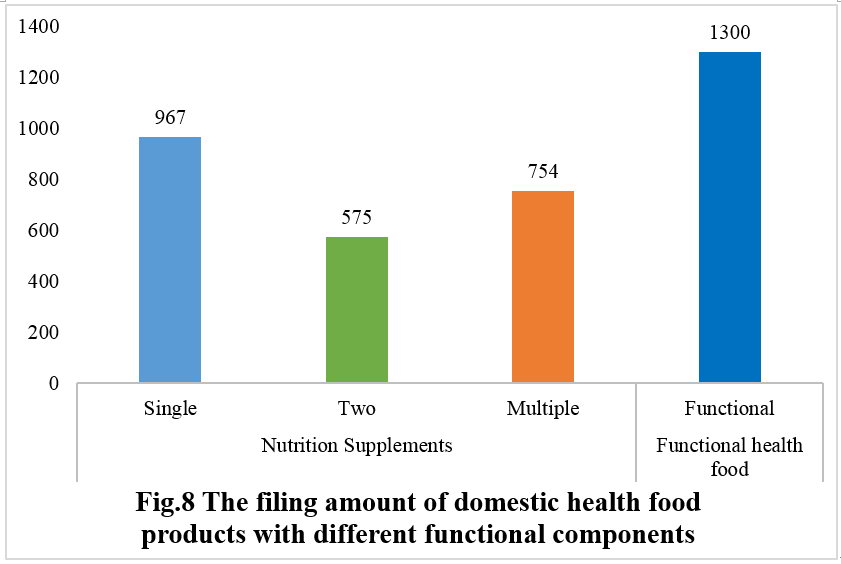
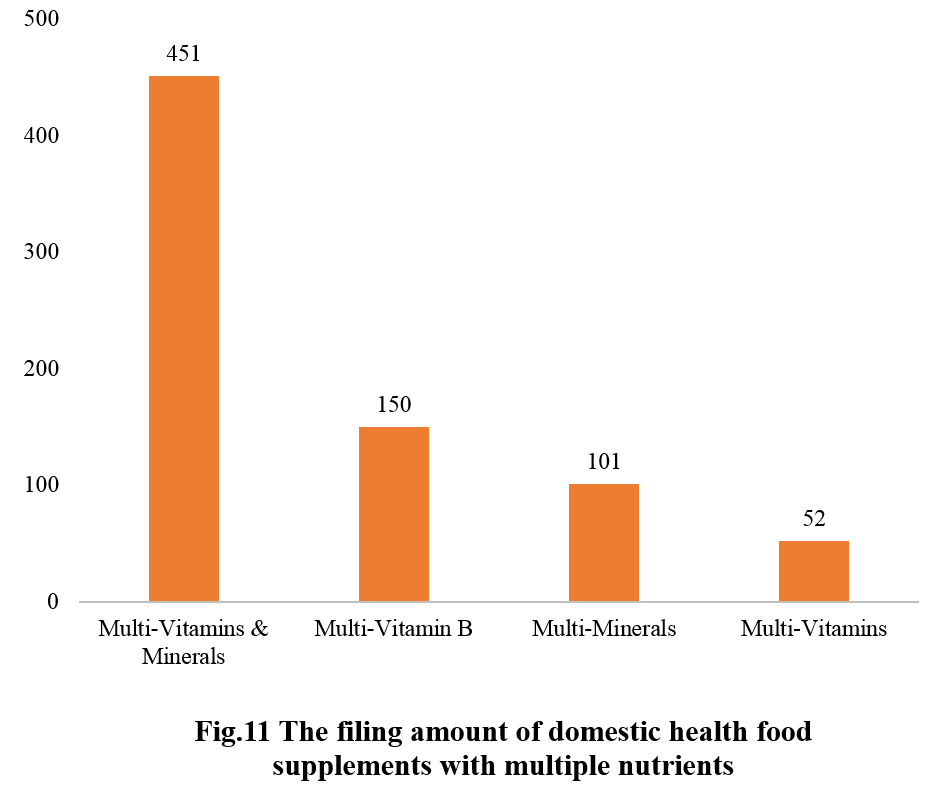
Among nutrition supplements, products with the function of supplementing a single nutrient are greater in quantity (967 products filed), followed by those supplementing multi-vitamins & minerals (754 products filed) and two nutrients (575 products filed).
Among nutrition supplements, the three major products are vitamin C supplements, calcium & vitamin D supplements, and multi-vitamins & minerals supplements. The filed quantities are 409, 234, and 451, respectively.
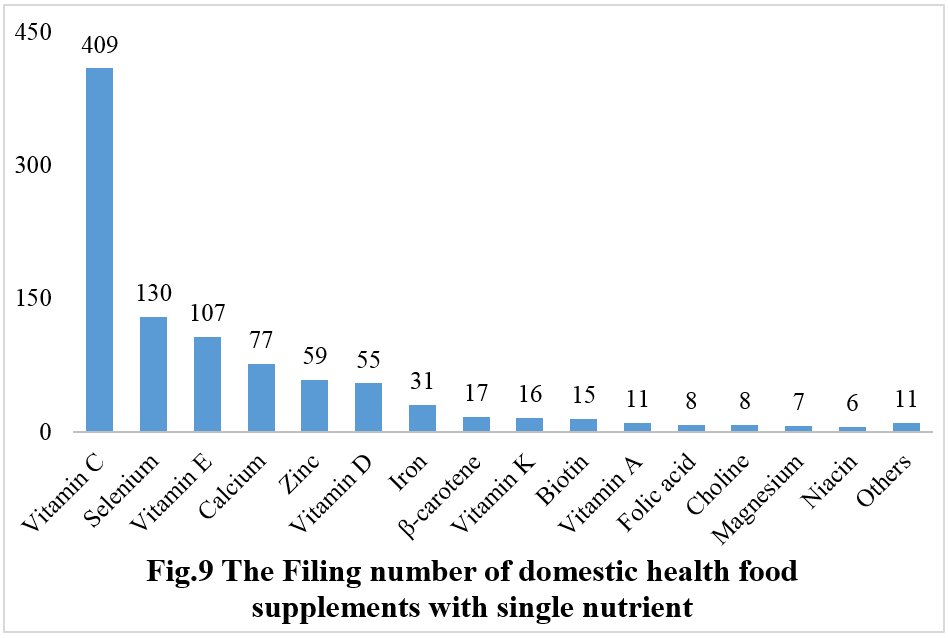
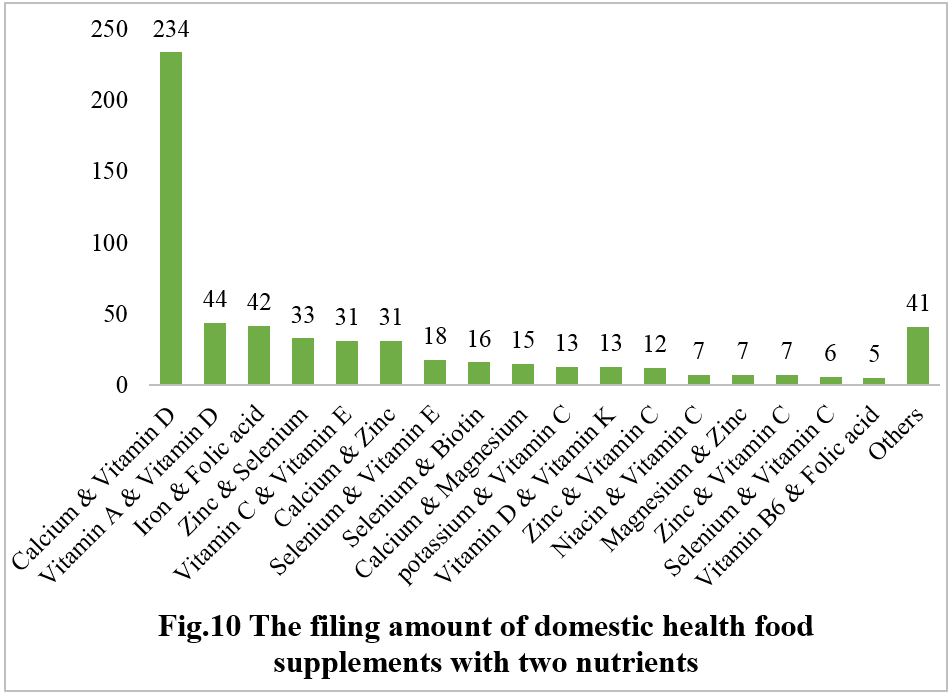
Note: Nutrition supplements with less than three products are classified as “Others”.
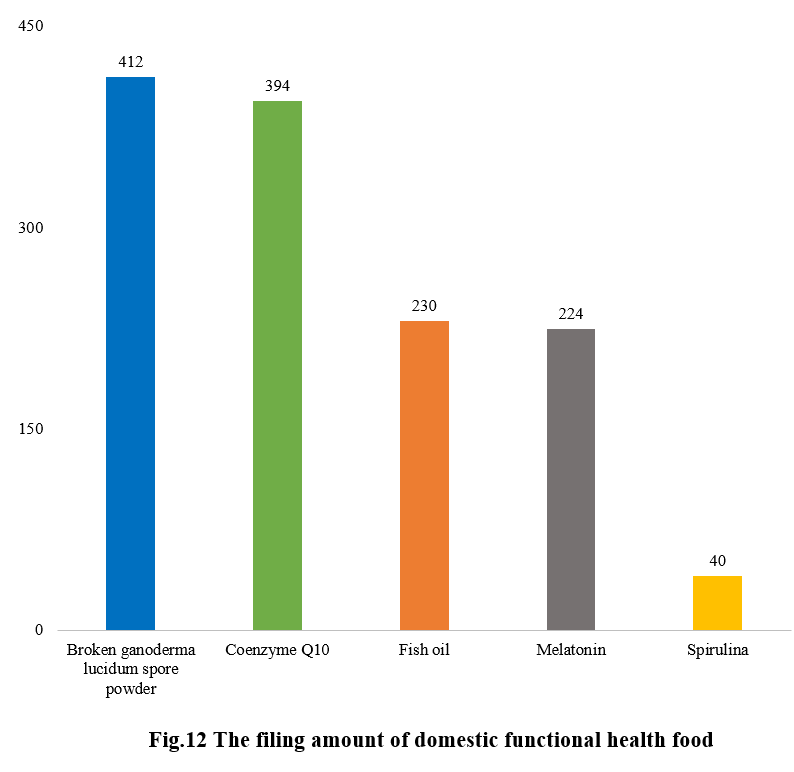
The functional health foods that have been filed all consist of health foods with broken ganoderma lucidum spore powder, coenzyme Q10, fish oil, melatonin, or spirulina as the single raw material. The filing numbers are 412, 394, 230, 224, and 40, respectively.
Imported health food
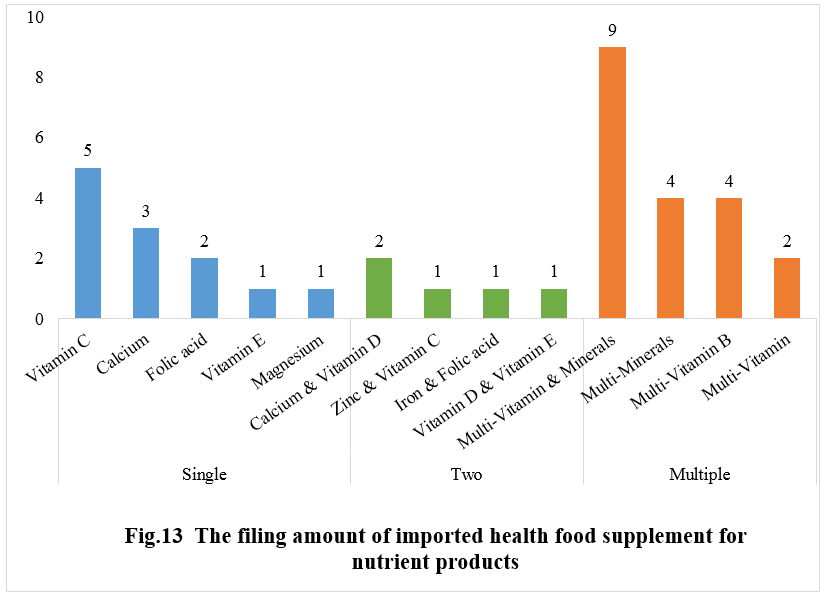
Among the imported health foods that have been filed in 2023, multi-vitamins & minerals supplements stand out as the mainstream products, with a total of 9 fillings. Following closely behind are Vitamin C supplements with 5 fillings, and multi-minerals and B-complex vitamin supplements, with 4 for each.
CIRS Opinion
On June 14, 2023, DHA, soy protein isolate and whey protein were added to the Directory of Health Food Raw Materials. On December 31, ginseng, American ginseng, and ganoderma lucidum were included, further expanding the Directory. Among the above raw materials, DHA is a new nutrient added to the Directory of Nutrient Supplements and its functions are supplementary nutrients, and imported enterprises can also apply for the filing of DHA products. On the other hand, soybean protein isolate, whey protein, ginseng, American ginseng, and ganoderma lucidum, similar to 5 raw materials including ganoderma lucidum spore powder, will remain to be limited to domestic products. It is anticipated that in 2024, with the continuous expansion of the Directory of Health Food Raw Materials, more health foods will enter the market. CIRS Group has launched ChinaFoodDB, a one-stop query platform for food raw materials and regulations launched by CIRS Group, aiming to provide enterprises and users with front-end compliance services, including food raw material inquiries, formula compliance self-checks, and access to various regulations and official announcements. It is believed to become a powerful tool for enterprises in the process of formula R&D and product innovation.
Note:
- The data in this article is sourced from the Special Food Information Query Platform.
- The release of data on the Special Food Information Query Platform may be subject to delays. The data in this article is for reference only, please refer to the official information.
If you need any assistance or have any questions, please get in touch with us via service@hfoushi.com.

