In accordance with the regulatory requirements in China, the health functions claimed by registered health food must be listed in the directory of health food functions. Currently, there are a total of 24 health functions listed in the directory. Related enterprises need to select functions that aligned with their products from the directory.
To further manage the health function claims, the State Administration for Market Regulation (SAMR) has issued and implemented the Administrative Measures on Health Food Raw Material Directory and Function Directory and Detailed Rules for Technical Review of New Functions and Products of Health Food (Trial), officially opening the channel for new health functions applications.
Current regulations
|
Regulations |
Date of implementation |
|
Administrative Measures on Health Food Raw Materials Directory and Function Directory |
2019.10.1 |
|
Detailed Rules for Technical Review of New Functions and Products of Health Food (Trial) |
2023.8.28 |
Qualification requirements for applicants
- Applicants can be any organization or individual; and
- Based on relevant research, any organization or individual can independently or jointly submit new functions applications to the Center for Food Evaluation of the State Administration for Market Regulation.
Requirements for new functions
The new functions for health food should be clear and categorized into the following three types:
- Supplementing dietary nutrients;
- Maintaining or improving body health; and
- Reducing risk factors for diseases.
Application processes
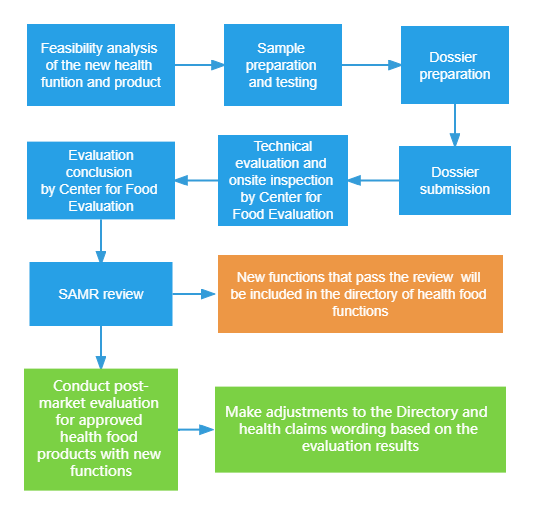
Note: If the applicant submits applications for a new function and registration of health food simultaneously, Center for Food Evaluation (CFE) should accept them at the same time and conduct associated evaluations.
Dossier requirements
For the application of new health functions:
Applicant shall provide technical evaluation materials according to the Detailed Rules. Specific materials items are as follows:
- Table of contents of the new health function;
- Letter of commitment for authenticity;
- Copies of the ID card or registration certificate of the applicant;
- Name, explanation, mechanism, and supporting evidence of the new health function;
- R&D reports of the new health function;
- Evaluation and verification materials of the new health function;
- Same or similar health function application status in China and other countries;
- Other scientific supporting evidence;
- Materials related to the ethics of health function;
- Technical evaluation of the sample;
- Other materials related to the function evaluation;
- Other evaluation materials related to human consumption; and
- Other issues that need to be specified.
For the registration of health products with new functions:
The materials shall be prepared according to the regulations of health food registration, and the applicant shall meet the qualification requirements.
Our Services
- Health Food Formula and Production Process Research and Development
- Domestic/Imported Health Food Registration
- Domestic/Imported Health Food Filing
- Domestic/Imported Health Food Registration Renewal
- Domestic/Imported Health Food Technology Transfer Registration
- New Health Food Functions Application
Further Information