Since the Administrative Measures for Health Food was implemented in June 1, 1996, China has approved 18,593 health food products in total by Dec. 31st 2021. The number of domestic and imported approved health food are 17,808 and 785 respectively. In order to help enterprises have an overview of this kind of products in China, CIRS counted the data of the approved health food registration products from 1996 to 2021 in the Special Food Information Query Platform of State Administration for Market Regulation (SAMR), and made an analysis for your reference.
1. Trends in the Number of Approved Health Food Registration
It can be seen in Fig.1, in 1997, the first year after the implementation of the Administrative Measures for Health Food, the number of domestic and imported approved health food has both increased significantly. And due to the institutional reform in 2018, the review process was stagnant, which resulted in only10 products being approved in that year. After the registration review of health food was reactivated, the number of approved health foods was increased in 2019 and 2020.
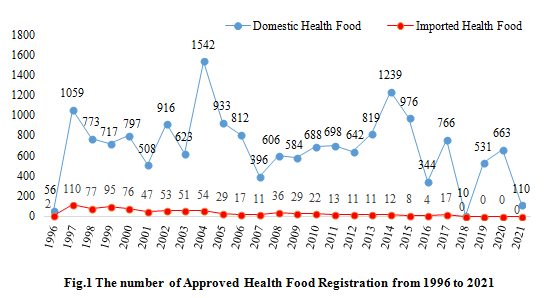
2. Analysis of Health Food Registration from 2015 to 2021
2.1 Proportion of Registrations of Functional Health Food and Nutrition Supplement
Vitamin and mineral nutrition supplements accounted for 38% and 31% of the total approved health food in 2015 and 2016, respectively. And all approved health food are nutrition supplements in 2017. In 2018, all approved health food are functional health food. In 2019 and 2020, functional health food accounted for 79.48% and 91.33% of the total approved health food, respectively, and some domestic nutrition supplements using raw materials out of the health food raw materials directory obtained registration certificates. Among the approved health food in 2021, 99.09% are functional health food, with one nutrition supplement product using raw materials out of the health food raw materials directory obtained registration certificate.
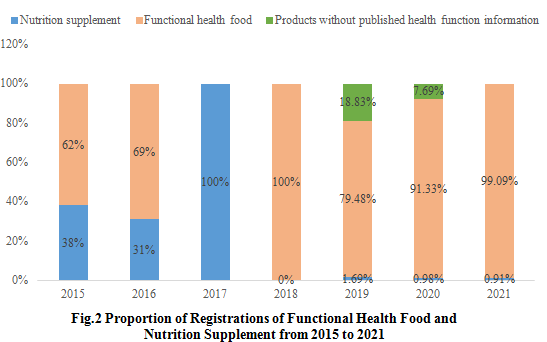
Note: Due to the lack of information in Special Food Information Query Platform, the health functions information of some products are not published for the moment.
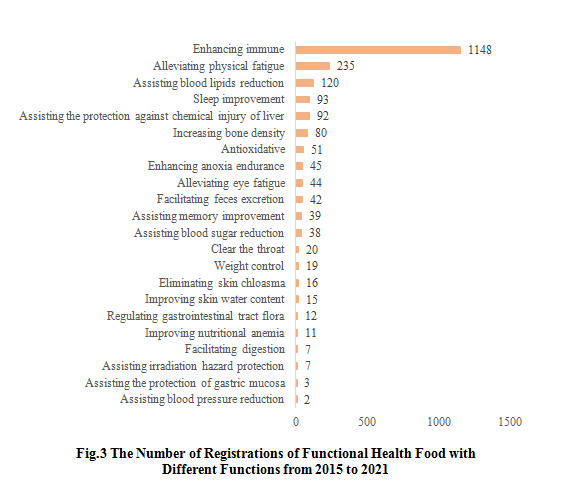
2.2 The Number of Registrations of Functional Health Food with Different Functions
From 2015 to 2021, there are a large number of approved health foods with enhancing immune and alleviating physical fatigue functions, which are 1148 and 235 respectively. CIRS speculates that the main reason for the high application volume is that the products with these two functions do not need to be tested on human body. On the other hand, the number of approved health foods with facilitating digestion, assisting irradiation hazard protection and assisting blood pressure reduction etc. are all less than 10. And the number of approved health foods with facilitating milk secretion, alleviating lead excretion, improving child growth, improving skin oil content, and eliminating acne functions is 0.
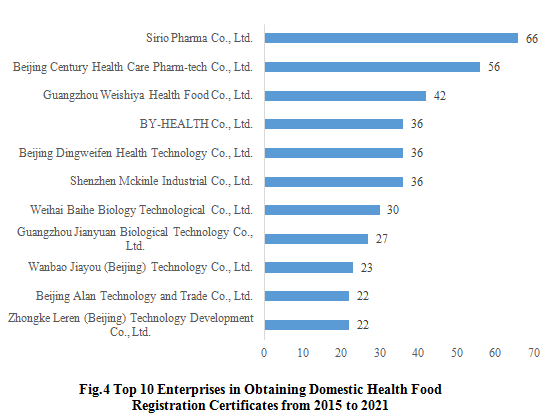
2.3 Enterprises Obtaining Health Food Registration Certificates
From 2015 to 2021, the domestic company, Sirio Pharma Co., Ltd., has got the largest number of registration certificates of health food, which is 66, followed by Beijing Century Health Care Pharm-tech Co., Ltd.
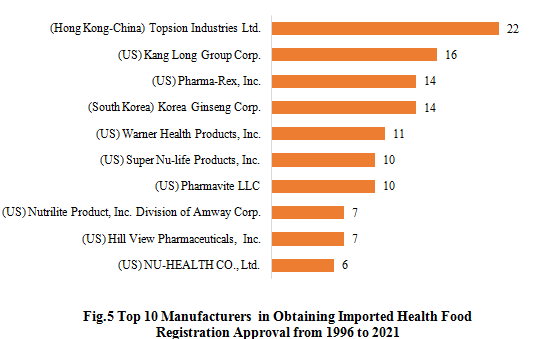
As only 29 imported health foods were approved from 2015 to 2021, the data is lack of statistical significance. Therefore, the statistical scope here is extended to 1996-2021 for imported health foods. Accosting to Fig. 5, Topsion Industries Ltd. has got the largest number of imported health food registration certificates from 1996 to 2021, which is 22, followed by Kang Long Group Corp.
3. CIRS Comments
After the abolishment of Technical Standards for Testing & Assessment of Health Food (2003 version) in the year of 2018, the registration tests of new products in China have been suspended, which hindered the application of new products in recent years, and also indirectly led to a decline in the number of approved products in recent years. At present, most test methods have been officially issued, and the functional assessment methods have been issued for public comments for the second time. It can be expected the official version of functional assessment methods would be released in 2022, so that enterprises can carry out the new products registration application smoothly.
If you have any needs or questions, please contact us at service@hfoushi.com.
Data Source
Special Food Information Query Platform of State Administration for Market Regulation (SAMR) and new approval announcements.

