Application of Genetically Modified Microorganism New Food Raw Material
In China, it is imperative to submit product safety evaluation materials to the National Health Commission (NHC) before producing or importing new food raw materials. However, when the production process involves genetically modified technology, the overall safety evaluation becomes a crucial issue.
Previously, China HNC only accepted the application of new genetically modified microorganism (GMM) enzymes. In 2021, the national regulatory authorities officially opened the application channel for GMM food additives, providing a broader scope for the research and application of food additives. In 2022, the National Development and Reform Commission (NDRC) issued the 14th Five-Year Plan for the Development of Bioeconomy, which clearly states the goal to expand and strengthen bioeconomy, with the aim of making it a strong driving force for high-quality development by 2025. Today, with the strong support of various favorable policies for synthetic biology technology, it is anticipated that the application pathway for GMM new food raw materials will soon be opened.
What is Genetically Modified Microorganism New Food Raw Material?
New food raw materials refer to items that posses the characteristics of food ingredients, meet the necessary nutritional requirements, are non-toxic and harmless, do not cause any acute, subacute, chronic, or other potential harm to human health, and are not part of the traditional dietary habits in China. The specific categories are as follows:
- Animals, plants and microorganisms;
- Components isolated from animals, plants, and microorganisms;
- Food components with altered original structures;
- Other newly developed food ingredients.
While GMM new food raw materials are generally recognized as new food raw materials produced by microorganisms using genetic engineering techniques to alter its genetic composition. Once genetic engineering technology is employed, such food raw materials are classified as “new food raw materials”.
The popularity of L-ergothioneine (EGT), an ingredient widely used in the field of cosmetics, in the functional food market abroad sets a vivid example. Currently, the mainstream production process involves synthetic biological fermentation. Without a doubt, it is considered a new food raw material in the food industry, whereas L-ergothioneine produced using genetic engineering technology is required to apply for genetically modified new food raw material.Likewise, another noteworthy substance is sialic acid, prevalent in infant formula, protein powder, and nutrition supplements for the elderly, which is also obtained through synthetic biological fermentation. Despite the prior approval by NHC back in 2017, it remains pertinent to underscore that sialic acid produced via synthetic biology mandates application as a genetically modified new food raw material..
What Are the Application Processes for Genetically Modified Microorganism New Food Raw Material?
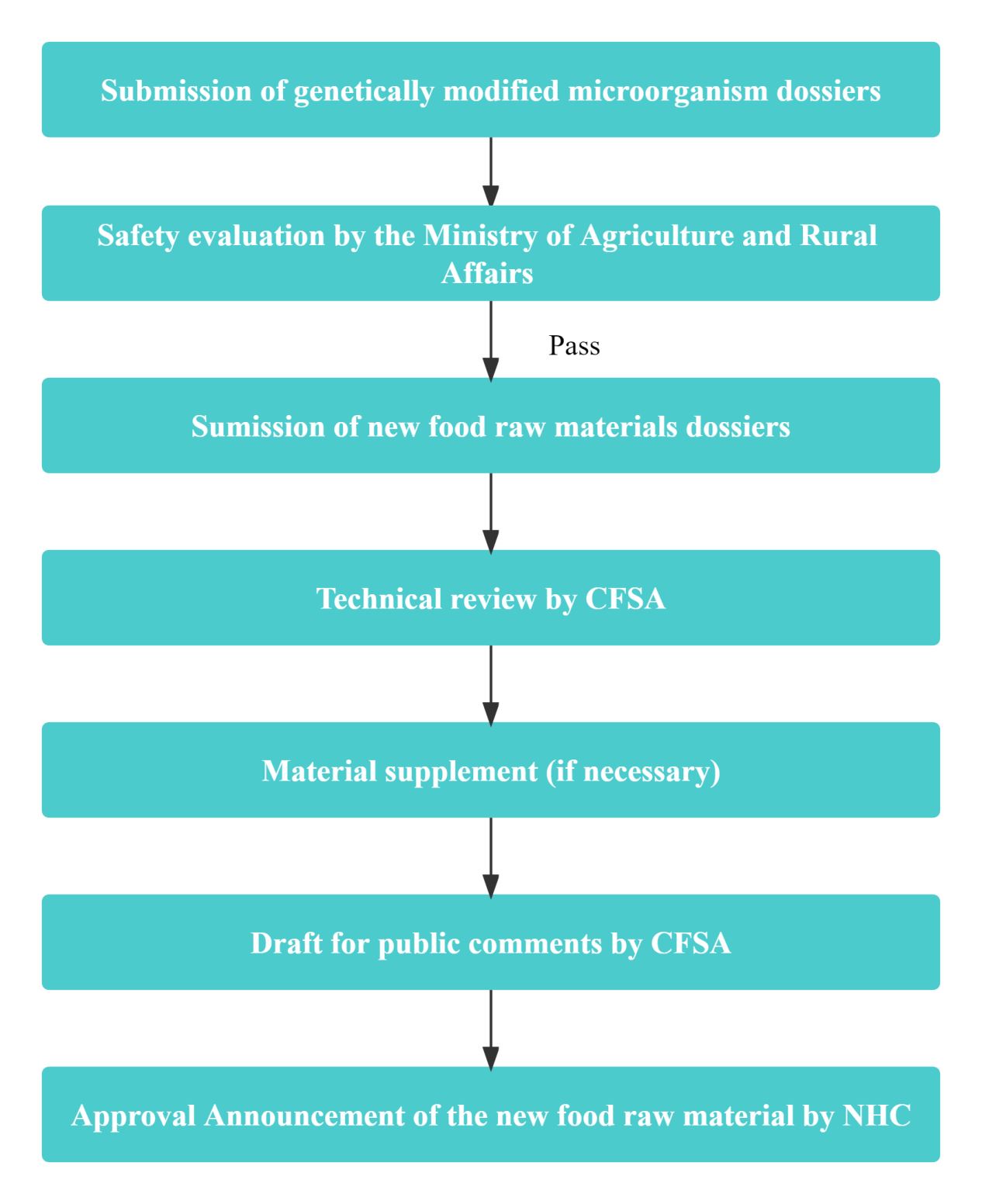
Compared to common new food raw materials, the application process for GMM new food raw materials is more intricate, involving evaluation or approvals from the following two regulatory authorities:
- Ministry of Agriculture and Rural Affairs (MARA): Responsible for conducting safety assessments of the genetically modified microorganisms;
- National Health Commission (NHC): Responsible for the evaluation and approval for the final products derived from the genetically modified microorganisms, also recognized as the new food raw materials.
What are the Dossier Requirements for Genetically Modified Microorganism New Food Raw Material Application?
The applicant is required to submit the following dossiers:
- Safety evaluation of the genetically modified microorganisms;
- Application dossiers of new food raw materials.
Our Strengths
The Food Division of CIRS Group boasts a professional technical team specializing in the field of “Three New Foods”. With extensive experience, we have made a track record of numerous successful cases in the regulatory application and filing of new food raw materials, new food additives and new food contact materials. Currently, CIRS Group has taken on the agency for the application of dozens of GMM new food raw materials and additives projects, positioning us at the forefront of the industry. We warmly welcome inquiries or visits for a more in-depth insight into our technological capabilities!