On October 7, the National Health Commission of the People’s Republic of China (NHC) issued an announcement (No. 8 of 2023) on the approval of 15 “Three New Foods”, including four new food raw materials, six new food additives, and five food-related products. Details are as follows:
New food raw materials (four types)
1. Peach gum
|
Name |
Peach gum |
|
|
Basic information |
Source: Prunus persica (L.) Batsch |
|
|
Brief introduction of the production process |
Prepared through picking, sorting, airing, cleaning, drying, and other processes by using the gum secreted from Prunus persica (L.) Batsch. |
|
|
Recommended intake |
≤30 grams per day (g/day) |
|
|
Quality requirements |
Crude polysaccharide, grams per 100g (g/100 g) |
≥ 60 |
|
Moisture, g/100 g |
≤ 15 |
|
|
Ash, g/100 g |
≤ 2 |
|
|
Other information |
|
|
|
Lead (Pb), milligrams per kilogram (mg/kg) |
≤ 0.5 |
|
|
Arsenic (As), mg/kg |
≤ 0.5 |
|
|
Ochratoxin A, μg/kg |
≤ 5.0 |
|
|
Coliforms, CFU/g |
≤ 100 |
|
|
Mould and Yeast, CFU/g |
≤ 150 |
|
|
Staphylococcus aureus, /25g |
0 |
|
|
Salmonella, /25g |
0 |
|
2. Tiger nut
|
Name |
Tiger nut |
|
Basic information |
Source: Cyperus esculentus L. var. sativus Boeck. Edible part: stem tubers |
|
Other information |
Food safety indicators shall comply with the current national food safety standard for nuts and seeds. |
3. Leuconostoc mesenteroides subsp. cremoris
|
Name |
Leuconostoc mesenteroides subsp. cremoris |
|
|
Other information |
|
|
|
Pb, (on dry basis), mg/kg |
≤ 1.0 |
|
|
As, (on dry basis), mg/kg |
≤ 1.5 |
|
|
Salmonella, /25g (mL) |
0 |
|
|
Staphylococcus aureus, /25g (mL) |
0 |
|
|
Listeria monocytogenes, /25g (mL) |
0 |
|
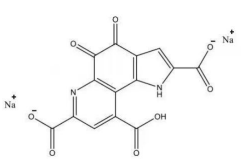
4. Pyrroloquinoline quinone disodium (PQQ) salt
|
Name |
Pyrroloquinoline quinone disodium (PQQ) salt |
|
|
Basic information |
CAS: 122628-50-6 Molecular formula: C14H4N2Na2O8 Structure:
Molecular weight: 374.17 |
|
|
Brief introduction of the production process |
Prepared through fermentation, extraction, purification, crystallization, drying, and other processes by using Methylovorus glucosotrophus as the production strain. |
|
|
Recommended intake |
≤20 mg/day |
|
|
Quality requirements |
Property: |
Reddish brown powder |
|
Pyrroloquinoline quinone disodium (PQQ) salt content (on dry basis), g/100 g |
≥ 98.0 |
|
|
Moisture, g/100 g |
≤ 12.0 |
|
|
Other information |
|
|
|
Pb, mg/kg |
≤ 0.5 |
|
|
As, mg/kg |
≤ 1.0 |
|
|
Cd, mg/kg |
≤ 0.1 |
|
|
Hg, mg/kg |
≤ 0.1 |
|
|
Total plate count, CFU/g |
≤ 1000 |
|
|
Coliforms, MPN/g |
≤ 3.0 |
|
|
Mould and Yeast, CFU/g |
≤ 100 |
|
|
Staphylococcus aureus, /25g |
0 |
|
|
Salmonella, /25g |
0 |
|
New food additives (six types)
1. New food enzyme (one type)
|
No. |
Enzyme |
Source |
Donor |
|
1 |
Serine protease |
Bacillus licheniformis |
Nocardiopsis prasina |
* The quality specifications of the enzyme shall meet the requirements in the National Food Safety Standard - Food Additives, Enzymes (GB 1886.174).
2. New food nutrition enhancers (three types)
(1) Name: Magnesium lactate
Application scope and maximum usage level: It shall be consistent with the requirements of magnesium in the National Food Safety Standard – Standard for the Use of Food Nutrition Enhancer (GB 14880).
Quality specifications: Applicable to the new food nutrition enhancer magnesium lactate, which is prepared through the reaction of lactic acid and magnesium oxide (or magnesium carbonate).
(2) Name: 2’-fucosyllactose, 2’-FL
Application scope and maximum usage level:
|
Category number |
Food name/category |
Usage level |
Note |
|
01.03.02 |
Modified milk powder (limited to milk powder for children) |
0.7-2.4 g/L (Count as the state of ready to eat, for powdery products, the level of use should be increased by times of brewing) |
When mixed with Lacto-N-neotetraose (LNnT), oligo-galactose, oligofructose, polyfructose and raffinose, the total amount of 2'-fucosyllactose must not exceed 64.5 (g/kg). |
|
13.01.01 |
Infant formula foods |
||
|
13.01.02 |
Older infants and young children formula foods |
||
|
13.01.03 |
Infant formula foods for special medical purposes |
Quality specifications: Applicable to the new food nutrition enhancer 2’-fucosyllactose (2’-FL), which is prepared through fermentation, purification, drying, and other processes using lactose as a raw material. The production strain of 2’-FL should undergo a safety assessment and comply with the requirements outlined in Appendix C.
Appendix C Information on the production strain of 2’-fucosyllactose
|
Nutrition enhancer |
Source |
Donor |
|
2’-fucosyllactose |
Coli K12 DH1 MDO |
(Helicobacter spp.)a |
|
E. coli K-12 MG1655 |
(Helicobacter spp.)a |
|
|
E. coli BL21 (DE3) |
(Neisseria spp.)a |
a is the donor of α-1, 2-fucosyltransferase
(3) Name: Lacto-N-neotetraose, LNnT
Application scope and maximum usage level:
|
Category number |
Food name/category |
Usage level |
Note |
|
01.03.02 |
Modified milk powder (limited to milk powder for children) |
0.2-0.6 g/L (Count as the state of ready to eat, for powdery products, the level of use should be increased by times of brewing) |
When mixed with 2’-fucosyllactose, oligo-galactose, oligofructose, polyfructose and raffinose, the total amount must not exceed 64.5 (g/kg). |
|
13.01.01 |
Infant formula foods |
||
|
13.01.02 |
Older infants and young children formula foods |
||
|
13.01.03 |
Infant formula foods for special medical purposes |
Quality specifications: Applicable to the new nutrition enhancer Lacto-N-neotetraose (LNnT), which is prepared through fermentation, purification, drying, and other processes using lactose as a raw material. The production strain of LNnT should undergo a safety assessment and comply with the requirements outlined in Appendix D.
Appendix D Information on the production strain of Lacto-N-neotetraose
|
Nutrition enhancer |
Source |
Donor |
|
Lacto-N-neotetraose |
E. coli K-12 DH1 MDO |
(Neisseria spp.)a and (Helicobacter spp.)b |
a is the donor of β-1,3-N-acetylglucosamine transferase
b is the donor of β-1,4-galactosyltransferase
3. New food additives with expanded scope (two types)
|
No. |
Name |
Function |
Category number |
Food name/category |
Maximum level (g/kg) |
Note |
|
1 |
Calcium lactate |
Stabilizer and coagulant, acidity regulator |
04.02.02.03 |
Pickled vegetables |
10.0 |
- |
|
04.02.02.04 |
Canned vegetables |
3.0 |
||||
|
2 |
Sanzan gum |
Thickener, stabilizer, and coagulant |
01.01.03 |
Modified milk |
0.5 |
- |
|
14.03.03 |
Mixed protein drinks |
0.75 |
Count as the state of ready-to-drink, increase the usage of solid drinks according to the dilution ratio. |
|||
|
14.08 |
Flavored drinks |
0.5 |
New food-related products (five types)
1. Food contact additives with expanded scope (three types)
|
No. |
Name |
CAS number |
Application scope |
Maximum level (%) |
|
1 |
C.I. pigment black 7; Carbon black |
1333-86-4 |
Plastic: PEEK |
0.5 |
|
2 |
Copolymer of acrylamide with methacryloyloxyethyltrimethylammonium chloride, itaconic acid, and N, N '- methylene bisacrylamide |
214495-32-6 |
Paper and paperboard |
1.5 (Count as the dry weight) |
|
3 |
2- (Vinyloxy) -1,2,3-propanecarboxylic acid tributylester |
77-90-7 |
Ink with indirect contact with food |
10 |
2. New food contact resin (two types)
|
No. |
Name |
CAS number |
Range of application |
Maximum level (%) |
|
1 |
1,4-Benzenedicarboxylic acid, polymer with sebacic acid and 1,2-ethanediol |
25067-21-4 |
Coating and coating film |
Appropriate level |
|
2 |
Polymers of methacrylic acid with butyl methacrylate, ethyl acrylate and methyl methacrylate, and polymers of hydroquinone with 4,4-methylenebis (2,6-dimethylphenol) and chloromethylepoxyethane with N, N-dimethylethanolamine |
- |
Coating and coating film |
20 (Count as the coating formula) |
If you need any assistance or have any questions, please get in touch with us via service@hfoushi.com.
Further Information
NHC News