In order to help enterprises better understand the filing status of health food, CIRS counted the data of filed products published in the first half of 2021 and made an analysis for your reference.
1. The Filing Status of Health Food
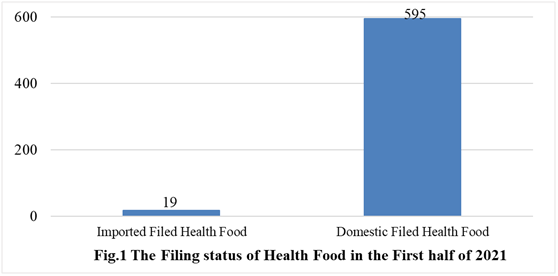
According to the information released by the Center for Food Evaluation of SAMR and the Local Administration for Market Regulation, a total of 614 health food obtained the filing certificates, of which 595 are domestic health foods and 19 are imported health foods by June 30, 2021.
2. The Filing Status of Health Food in Different Regions
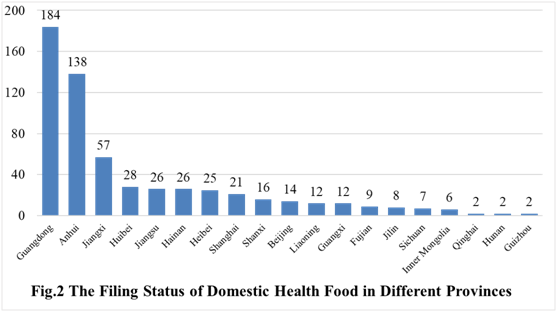
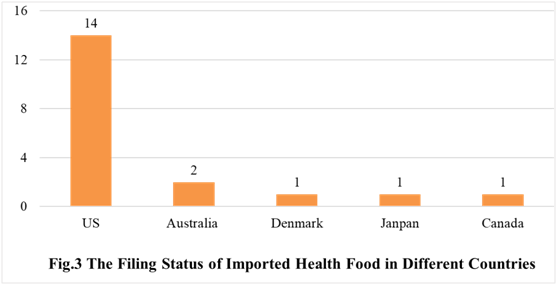
The number of approved domestic filing health food are varied in different provinces in China. Guangdong and Anhui rank the first and second place respectively with the number of 184 and 138. The applicants who come from the United States, Australia, Denmark, Japan and Canada obtained the filing certificates of imported health food. There are 19 imported filing products, and the applicants of 14 products are US enterprises.
3. The Filing Status of Health Food in Different Enterprises
3.1 Domestic Filing Products
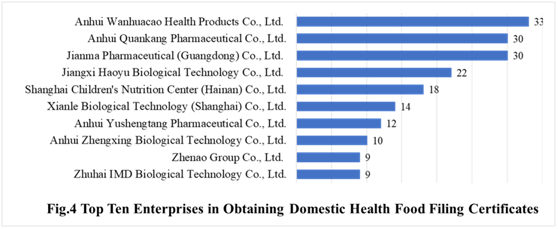
In the first half of 2021, there are 169 domestic health food manufacturers who have obtained health food filing certificates. Anhui Wanhuacao Health Products Co., Ltd. has the largest number of filings, and a total of 33 products obtained the filing certificates, followed by Anhui Quankang Pharmaceutical Co., Ltd. and Jianma Pharmaceutical (Guangdong) Co., Ltd. with the number of 30 and 30, respectively.
3.2 Imported Filing Products
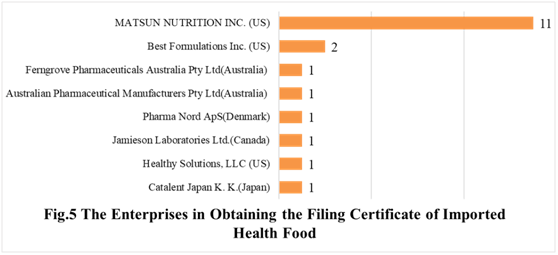
In the first half of 2021, there are 9 oversea companies who have got health food filing certificates. MATSUN NUTRITION INC. has the largest number of filings, and a total of 11 products obtained the filing certificates. Mega Health International, Inc. ranked second place with the number of 2 products.
4. The Filing Status of Health Food in Different Dosage Forms
At present, the permitted dosage forms of Chinese health food products include tablets, capsules (hard/soft), oral liquids, granules, powders and gelatin candies.
4.1 Filing status of domestic products
In the first half of 2021, the main dosage form for domestic health food filing is tablets with the number of 344, accounting for 57.81% of the total.
The capsules include soft capsules and hard capsules, and the number of filings is 164. The quantity of soft capsule products is far higher than the number of hard capsule products, which are 150 and 14 products respectively.
In addition, the quantity of oral liquid (including drops) and granule products are 35 and 49 respectively. Among them, there are only 3 drops products.
Powders and gelatin candies are the new dosage forms available for filing since July 1, 2021, and 3 products in these 2 dosage forms have got the filing certificates. Detailed information are shown in Table 1
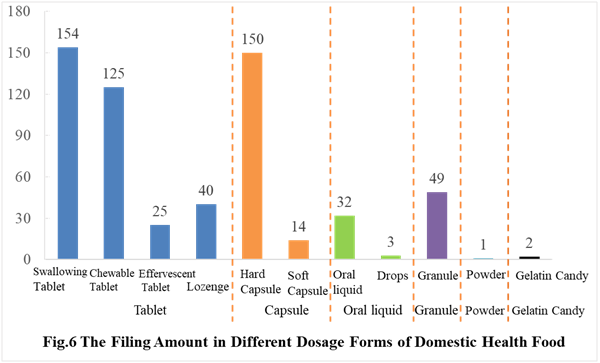
Table 1 The filing information of gelation candy and powder
|
Product Name |
Dosage Form |
Filed Enterpris e |
|
Cuiyi® Calcium Gel Candy (Blueberry Flavor) |
Gelatin Candy |
Xianle Health Technology (Anhui) Co., Ltd |
|
Cuiyi® Calcium Gel Candy |
Gelatin Candy |
Xianle Health Technology (Anhui) Co., Ltd |
|
Life Winner® Broken Ganoderma Spore Powder. |
Powder |
Shaanxi Dazhi Pharmaceutical Co., Ltd. |
4.2 Filing status of imported products
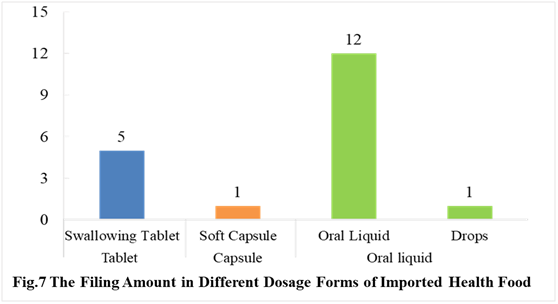
In the first half of 2021, the dosage form of approved imported health foods is mainly oral liquids (including drops) with the number of 13. The numbers of tablet and capsule product are 5 and 1 respectively. There is no powder, gelatin candy and granular products in the first half of 2021.
5. The Number of Approved Filed Health Food with Different Nutrients
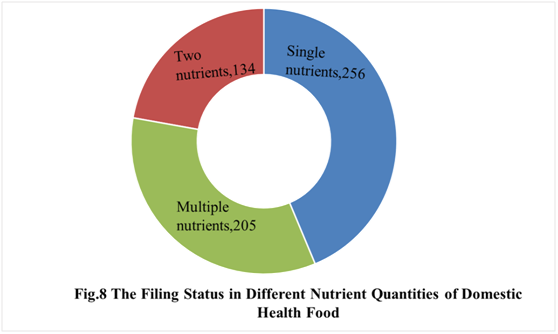
In the first half of 2021, the number of domestic products supplementing single nutrient is the most, which is 256, followed by the products supplementing multiple nutrients and two nutrients, the number are 205 and 134 respectively.
Among the domestically filed products in the first half of 2021, the most popular nutrition supplements are Vitamin C supplements, Calcium and Vitamin D supplements, multi-vitamins and minerals supplements, which is 96, 40 and 122 respectively.
Coenzyme Q10, Ganoderma lucidum spore powder and melatonin products were transferred from registration to filing supervision for the first time, and the filing numbers are 2, 1, and 1, respectively. The detailed information of these functional products are shown in Table 2.
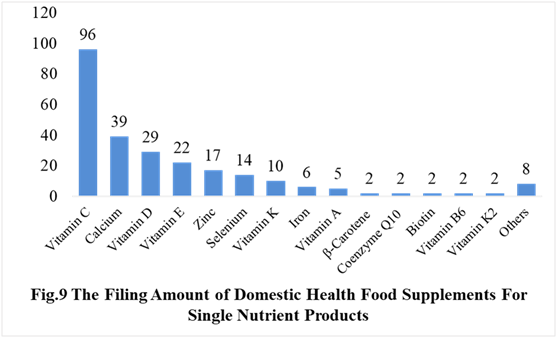
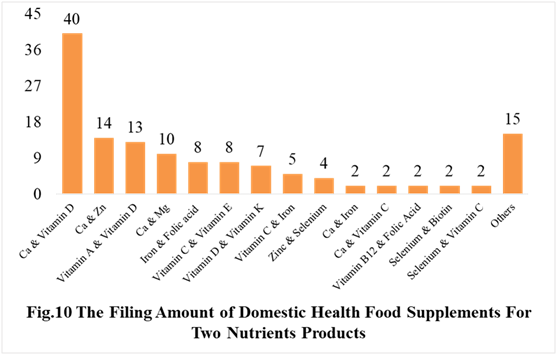
PS. Nutrients with less than 2 products are classified as “Other”.
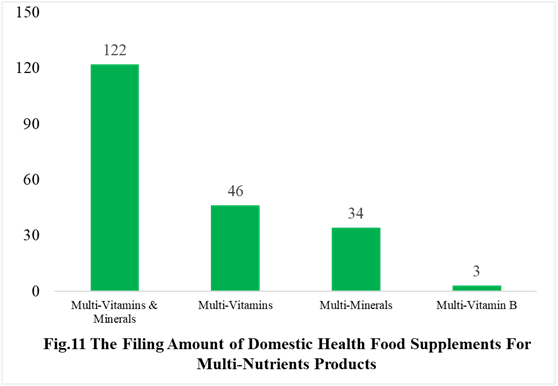
Table2 The filing information of functional products
|
Types of nutrients |
Health function |
Filed Enterprise |
|
|
Lingqu brand coenzyme Q10 soft capsules |
Coenzyme Q10 |
Enhancing immune |
Shaanxi Shenlong Pharmaceutical Co., Ltd. |
|
Tianci'an brand coenzyme Q10 capsules |
Coenzyme Q10 |
Enhancing immune |
Sanyuan Huazhou Pharmaceutical Bioengineering Production Base |
|
Hanlian brand melatonin tablets |
Melatonin |
Sleep improvement |
Shaanxi Shenlong Pharmaceutical Co., Ltd. |
|
Ganoderma lucidum spore powder |
Enhancing immune |
Shaanxi Dazhi Pharmaceutical Co., Ltd. |
5.2 Imported Filing Products
In the first half of 2021, the number of imported products supplementing multi-vitamins and minerals is the most, which is 6, followed by the products supplementing iron and Vitamin D, and other nutrients, which is 2 and 1 respectively.
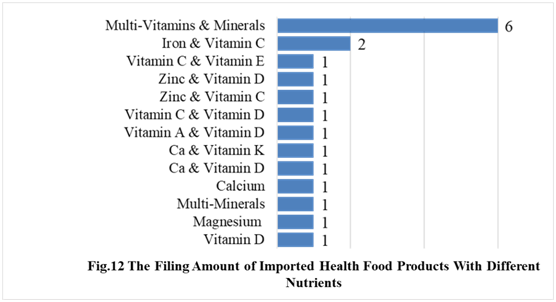
CIRS Comments
Starting from June 1, 2021, domestic health foods that use Coenzyme Q10, Broken Ganoderma lucidum spore powder, spirulina, fish oil or melatonin as single raw materials can apply for filing in China, and three products have got the filing certificates rapidly. At the same time, gelatin candies and powders have become the approved dosage form for fiing products. The filed products of two new dosage forms, gelatin candy and powder, appeared rapidly in the first half of 2021. Companies have gained more space in the selection of raw materials, function claims and dosage forms for filing products due to the implementation of these regulations. However, it should be noted that imported products made from five single raw materials such as Coenzyme Q10 still need to apply for registration.
If you have any needs or questions, please contact us at service@hfoushi.com.
Note:
I. The data in this article is from the Special Food Information Query Platform, Center for Food Evaluation of SAMR, and Local Administration for Market Regulation.
II. There may be some omissions in the data of domestic filed health food due to the replacement of new and old websites of government departments after the reform of state institutions, thus the data in this article is for reference only, and please refer to the information published by the government.
III. The Special Food Information Query Platform and Center for Food Evaluation of SAMR lag behind in information release. According to CIRS’s knowledge, the information of some products that have obtained health food filing certificates have not been published on the official website of the government departments. Therefore, the actual record amount of health food exceeds the data listed in this article.
