8 June 2016, China Food and Drug Administration ( CFDA) released the formal version of Administrative Measures on Product Formula Registration of Infant Formula Milk Powder , which will come into force on 1 October 2016. Based on the analysis of CIRS, there are not many differences between the formal version and the one submitted to WTO before. Meanwhile, the allowed quantity of registered formula that has much concern by the public has been official stipulated in this measure.
Application Scope
This regulation is applicable for the registration of product formula of infant formula milk powder which is produced and distributed in China and imported to China.
What is the product formula of infant formula milk powder?
The product formula of infant formula milk powder means all the raw materials, food additives and their dosages, and the product’s nutrition content.
Who should apply for the registration of product formula of infant formula milk powder?
The applicant should be the manufacturer of the infant formula milk powder in China or the overseas manufacturer plan to export infant formula milk powder to China.
Who should be responsible for the management of the registration of product formula of infant formula milk powder?
CFDA acceptance agency, evaluation agency, and examination agency should be responsible for the acceptance, evaluation, and on-site inspection of registration of product formula of infant formula milk powder, respectively.
What are the required materials?
-
Application form
-
Qualification certificate of applicant.
-
Quality and safety standards of raw materials
-
Formula research report
-
Production process specification
-
Product testing report
-
Evidentiary materials of the production, research and testing capabilities
-
Other materials to prove the scientificity and safety of the formula
How many formulas could be registered by one company?
-
There should have a significant difference between each registered product formula which is produced by the same company and for the same age. It should be confirmed by scientific basis, and there are at most 3 series 9 kinds of formulas for one company in principle. One series includes infant formula (0-6 months, 1 stage), older infant formula (6-12 months, 2 stage), and young children formula (12-36 months, 3 stage).
-
One wholly-owned subsidiary who has get the formula registration license and production license is allowed to produce the registered product from another wholly-owned subsidiary in the same group company.
Contents of the certificate
-
Product name
-
Company name, legal representative, and production address
-
Approval number, approval date and validity period
-
Production process
-
Product formula
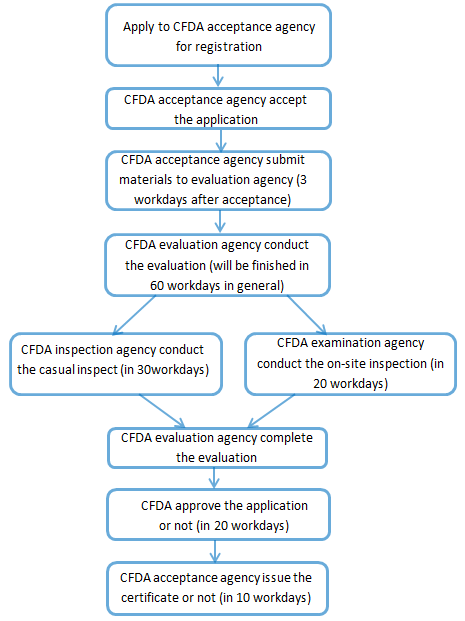
Registration procedure of the formula of infant formula milk powder
Forbidden content on the label and specification
-
Express and imply with the function of disease prevention or treatment.
-
Express and imply with health function.
-
Express and imply with the function of improving intelligence, strengthening resistance or immunity, protecting intestine, etc.
-
Words like ‘zero- added’, ‘no added’, ‘do not contain’ for the materials that are not allowed to add in the product in accordance with relevant food safety standard.
-
Contents that are false, exaggerated, violating the principles of science or absolute.
-
Other claims that are inconsistent with the product formula registration content.
The formal regulation will come into force on 1 October and related enterprises should get ready to apply for the registration by collating required materials etc. CIRS will keep in touch with authority and focus on the new updates in the following days since the registration details have not been published. Any progress, we will inform on the website immediately for timely response.
If you have any other questions, please contact us at service@hfoushi.com.
For further information of regulatory compliance on infant formula milk powder, please click here.
Reference
http://www.sfda.gov.cn/WS01/CL0053/155260.html
https://m.hfoushi.com/en/food/infant-formula-milk-powder-registration-in-china

