On 10 April 2018, State Administration for Market Regulation (hereinafter referred to as SAMR) issued a Notice on the Supervision of Food and Drug during the SAMR and State Administration for Drug Regulation (hereinafter referred to as SADR) Institutional Reform Period.
1. The main content of the Notice: before the “Three Legalizations” issued, the supervision for special food remain unchanged
According to the Decision of Central Committee of the Communist Party of China about Deepening the Institutional Reform of Party and State Institutions and the Decision of the First Session of the Thirteenth National People’s Congress on the Restructure Program of the State Council, the SAMR will be established as the department directly under the State Council; the SADR will be established and administered by SAMR, and the CFDA will be abolished. At present, the institutional reform work is being carried out.
Before the “Three Legalizations” (determine institution, determine function, and determine authorized strength ) plan of SAMR and the SADR issued, the matters undertaken by the former CFDA are still handled according to the original regulations, including the review, approval, supervision, inspection, law enforcement, complaints report and information disclosure for food, health food, infant formula milk powder, foods for special medical purpose (FSMP), medicines, medical devices and cosmetics; Meanwhile, all kinds of approval documents, certificates and official dispatches are in the original format, the business seal and text format remain unchanged, and the procedures remain unchanged as well.
After the institutional reform is in place, relevant matters will be further informed.
2. The schedule for institutional reform
Zhang Mao, director of the SAMR, mentioned the main steps and schedule of the institutional reform in 2018 at the meeting of the SAMR. The main information is as follows:
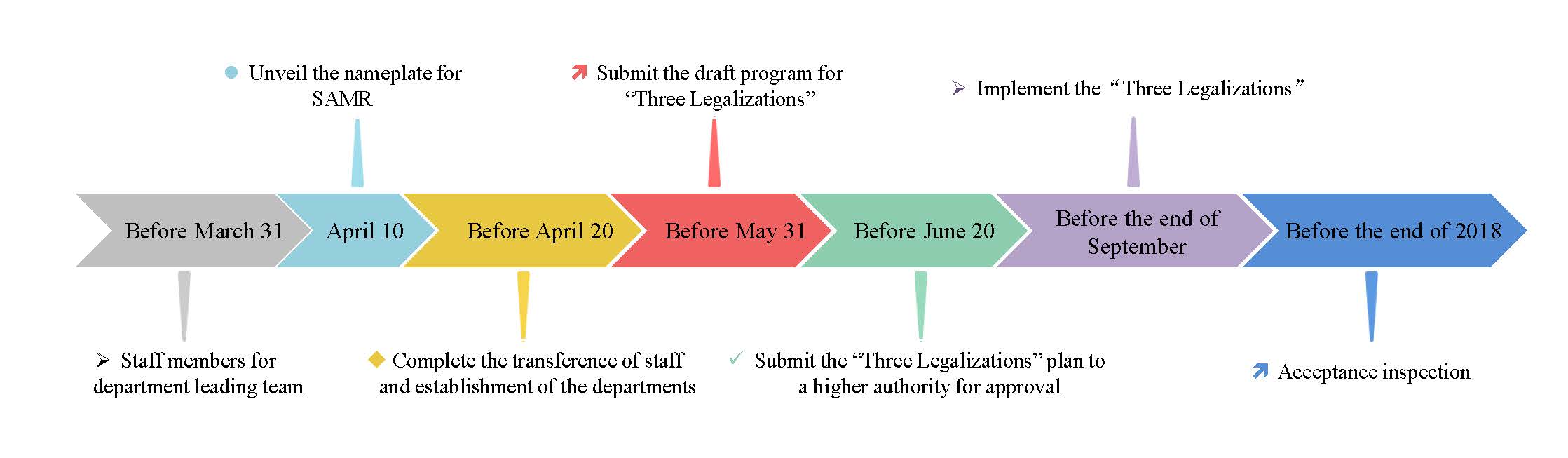
3. The effects of institutional reform on the supervision for special foods
According to the Notice, we can see that the supervision for special foods including health food, infant formula milk powder, and FSMP will remain unchanged before the “Three Legalizations” plan implemented (before the end of September, 2018).
After the “Three Legalizations” plan implemented, the SAMR will establish relevant departments to replace the former CFDA to be in charge of special foods supervision, however, the policies for special foods will remain unchanged for the moment.
Reference
http://samr.saic.gov.cn/xw/yw/zj/201804/t20180410_273684.html
