On-site inspection is a necessary step before FSMP products enter the Chinese market. It is one of the important basis for determining whether the applicant is qualified as a FSMP manufacturer through the strict and meticulous examination and investigation by the inspection experts. FSMP, however, is still a new food category in China: it has only been five years since the establishment of its registration system stipulated in Food Safety Law, and it has been less than three years since the first product was approved for registration. As a result, most enterprises still remain confused about the on-site inspection of FSMP. Therefore, CIRS is going to take enterprises to get through it based on relevant laws, standards and regulations.
Classification of FSMP On-site Inspection
According to Food Safety Law, Administrative Measures for Registration of Food for Special Medical Purposes and General Rules for Examination of Food Production License, the classification of on-site inspection of FSMP can be summarized as follows:
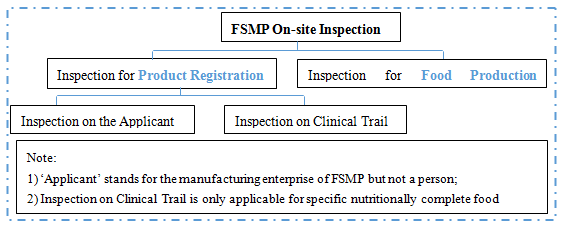
Details of Inspection for Products Registration
1. Who carries out the inspection?
Administrative Measures for Registration of Food for Special Medical Purposes (hereinafter referred as ‘The Measures’) states that, CFDA Inspection Institution (now referred to SAMR Food Review Center) is responsible for the on-site inspection for registration, and shall inform the provincial food safety regulatory department where the applicant is located to participate in the inspection. In short, the nation carries out and the province take part in for the registration on-site inspection.
2. How long the inspection takes?
The Measures states that, the Inspection Institution shall 1) complete the on-site inspection on the applicant and issue the inspection report within 20 working days from the date of receiving the notification and 2) complete the on-site inspection on clinical trial and issue the inspection report within 40 working days from the date of receiving the notification.
3. What the inspection consists of?
The inspection is divided by Major Points and Judgment Principles of On-site Inspection on Manufacturer of Food for Special Medical Purposes (hereinafter referred as ‘The Points’) into 8 parts which are:
1) Production capacity, 2) R&D capacity, 3) testing capacity, 4) production site, 5) equipment and facilities, 6) personnel, 7) material management, 8) production process management
The 8 inspection parts are further divided into 24 inspection items, among the following 5 are the key inspection items:
1) Qualification of the manufacturer, 2) R&D capacity, 3) production quality management system establishment, 4) production system, 5) production water
Meanwhile, other items related to the submitted application materials may be inspected based on the needs of technical review.
For on-site inspection of clinical trials, The Measures only states that ‘on-site inspection should be conducted on the authenticity, completeness, accuracy and other conditions of clinical trials’, and there are no more specific provisions.
4. How to determine the inspection result?
Among the 24 inspection items, the results of the key items are either ‘consistent’ or ‘inconsistent’, and the results of the other 19 items are ‘consistent’, ‘basically consistent’ and ‘inconsistent’. According to the summary of the results, the following decisions will be made:
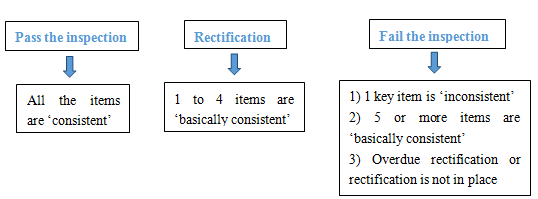
Rectification should be completed within 10 days. When the applicant thinks that the rectification is completed, the local provincial food safety supervision and administration department shall verify, confirm and sign, and the inspection institution shall make a decision of passing the on-site inspection.
Details of Inspection for Food Production License
1. Who carries out the inspection? How long the inspection takes?
Administrative Measures for Food Production License stipulates that, the administration of production license of FMSP is the responsibility of the market supervision and administration departments of provinces, autonomous regions and municipalities directly under the Central Government. General Rules for Food Production License Examination ( hereinafter referred as ‘The Rules’) stipulates that, the food safety supervision and administration department being responsible for daily supervision of the applicant shall send observers to participate in the on-site inspection, but shall not be a member of the inspection team to participate in the scoring and judgment of the inspection; Observers who have objections to on-site inspection procedures and results may report in writing to the licensing authorities within 3 working days after the completion of on-site inspection.
In shorts: provincial departments shall be responsible for on-site inspection and the departments being responsible for routine supervision of the applicant shall participate in.
The Rules states also that, the inspection shall be completed within 10 working days from the date of accepting the task.
2. What the inspection consists of? In what ways it is carried out?
The Rules states that, on-site inspection for food production license are mainly six parts:
1) Production site, 2) equipment and facilities, 3) equipment layout and processing techniques, 4) personnel management, 5) management system and its implementation, and 6) test report of trial-produced sample
Detailed Rules for FSMP Production License Examination makes the six parts more detailed to be adapted for FSMP.
As for the inspection methods, The Rules stipulates that, it shall be carried out by means of inspecting the production site, consulting relevant documents/materials and questioning relevant personnel . When necessary, the inspection team may conduct spot check and examination on food safety management personnel and professional and technical personnel of the applicant.
3. How to determine the inspection result?
There is also a scoring table for on-site inspection of food production license, which is Food and Food Additive Production License On-site Inspection Scoring Record Table (hereinafter referred as ‘The Table’). The Table divides the six parts into 34 inspection items, in which
1) ‘Test report of trial-produced sample’ is listed as one inspection item, and is scored as ‘1, 0.5, 0’;
2) The remaining 5 parts are divided into 33 items, and are scored as ‘3, 1, 0’.
The Table stipulates that, if there is no item scored as ‘0’ and the total score is or higher than 85, it shall be judged as passing the inspection; if there are ‘0’ items or the total score is less than 85, it will be judged as failing the inspection.
The following situation are judged as failing the inspection as well:
1) The applicant does not cooperate with the inspection;
2) Equipment and facilities cannot operate normally during the inspection;
3) The applicant conceals relevant information or provides false application materials;
4) Other circumstances in which inspection cannot be carried out normally due to subjective reasons of the applicant.
4. Rectification and examination
The Rules states that, the applicant shall rectify the problems found in the inspection within 1 month if the applicant passes the inspection, and submit the rectification result to the food safety supervision and administration department being responsible for daily supervision of the applicant. Meanwhile, the department shall conduct a re-inspection to the applicant within three months, and shall focus on whether the problems found in the on-site inspection have been rectified.
Last but not least
It shall be noticed that, for overseas applicant, 1) the time limit for on-site inspection on product registration would be determined according to the actual situation; 2) since there is no need to take the on-site inspection on food production license, a copy of qualification certificate of the overseas applicant as an FSMP manufacturer shall be provided.
Overall, both the on-site inspection for product registration and production license are based on the technical review of the application materials. The main purpose of on-site inspection is to verify whether the applicant's actual situation is consistent with the application materials, whether it is true, whether it is matched with the products applied for registration and whether it meets the requirements of relevant laws and regulations, and so to determine that if the applicant is qualified to be a FSMP manufacturer.
If you have any needs or questions, please contact us at service@hfoushi.com.

