Recently, CIRS Group once again successfully helped clients complete a regular registration under MEE Order No. 12. And the results have been notified on the MEE website.
China's main chemical regulation – the Measures for the Environmental Management Registration of New Chemical Substances (also known as MEE Order 12) – which came into effect on January 1, 2021, mainly focuses on new chemical substances that may pose higher risks to the environment and human health.
Compared with its predecessor – MEP Order 7 – the application requirements and review standards under MEE Order 12 are much stricter. It has been nearly two years since Order 12 took effect, yet only ten batches of regular registrations have been approved and notified, far less than the number of regular registration applications submitted by enterprises.
At the moment, it is quite difficult for most of the applicants of regular registrations to submit the application materials required, satisfy the requirements of formal examination and technical review, and finally, receive approval for registration by the competent authority.
As we recently successfully assisted a client in completing another regular registration we wanted to take this opportunity to share some of our experiences in this article.
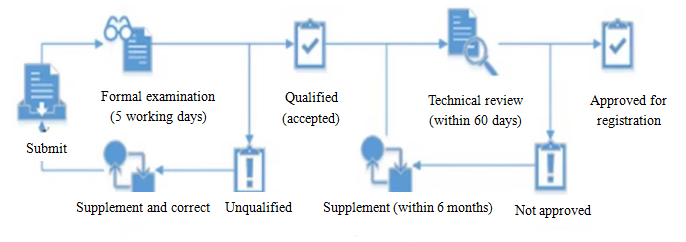
Regular registration reviews under MEE Order 12 mainly cover formal examination and technical review, which must be done by the Expert Committee (providing technical support) and the technique institutions for environmental management of chemical substances (undertaking the actual review work). The process of review is as follows:
The substance approved for registration this time is not classified as a persistent (non-P) and bio-accumulative (non-B) substance. As a toxic substance, it has undergone several formal examinations and technical reviews. Below is the registration experience from CIRS Group:
New chemical substances that are not highly hazardous (such as non-P and non-B) may have a higher priority for approval.
During the formal examinations, applicants should pay higher attention to whether the forms of the application materials conform to the specifications, or whether the testing data is complete. In addition, there are specific requirements for the qualification of both domestic and overseas testing institutions under MEE Order 12, and applicants are required to provide valid qualification documents from the testing institutions as well.
During the technical review, it is quite important to provide an environmental risk assessment report of high quality. Chemical Exposure Assessment Tool (CET) is usually used to conduct exposure assessments. Experts’ opinion mainly focuses on:
- The accuracy and rigorousness of statements;
- The applicability of the assessment basis;
- Quality of data;
- Selection of key parameters;
- The scientificity of hazard identification methods and parameters;
- The rationality and systematicness of emission scenarios;
- The setting basis for environmental exposure parameters;
- The application of assessment models;
- The comprehensiveness and systematicness of the generation, discharge, and destination of three wastes containing the applied substance; and
- The feasibility and pertinence of environmental risk control measures, etc.
CIRS Group suggests that related enterprises must collect complete information to conduct data gap analysis, reasonable tests, and determination of the persistence and bioaccumulation properties of substances when processing regular registrations. To complete the registration in a quick manner, applicants must provide reliable and scientific materials and documents, and make supplements and corrections promptly based on the opinions of the experts.
If you have any questions, please contact us at service@hfoushi.com.






