In order to help enterprises better understand the filing status of health food (dietary supplement) in China, CIRS made statistics on the health food approved in 2022 (as of July 5) and made an analysis from multiple perspectives.
1. The Filing Status of Health Food in China
According to the information released by the Special Food Information Query Platform, in 2022 (as of July 5), a total of 1483 health food obtained the filing certificates, of which 1481 are domestic health foods and 2 are imported health foods (detailed information is shown in Table 1).
Table 1. The Filing Information of Imported Health Food
|
Product Name |
Filing Number |
Applicant |
Country |
|
Senior Multivitamin Caplet |
食健备J202200000001 |
Jamieson Laboratories Ltd. |
Canada |
|
Healthy Care Kids Chewable Zinc+ Vitamin C |
食健备J202200000002 |
Nature’s Care Manufacture Pty Limited |
Australia |
2. The Filing Status of Health Food in Different Regions
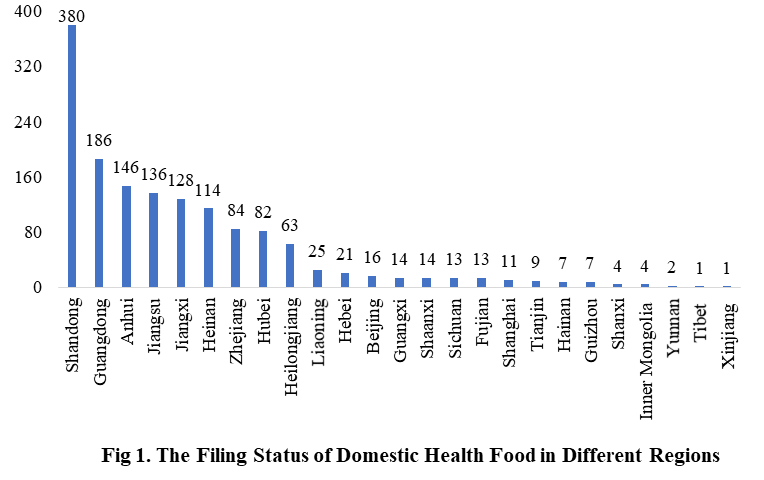
The number of approved domestic filing health food is varied in different regions in China. Shandong has the largest number of filings, and a total of 380 products obtained the filing certificates. Anhui and Guangdong rank the second and third place respectively with the number of 186 and 146.
3. The Filing Status of Health Food in Different Enterprises
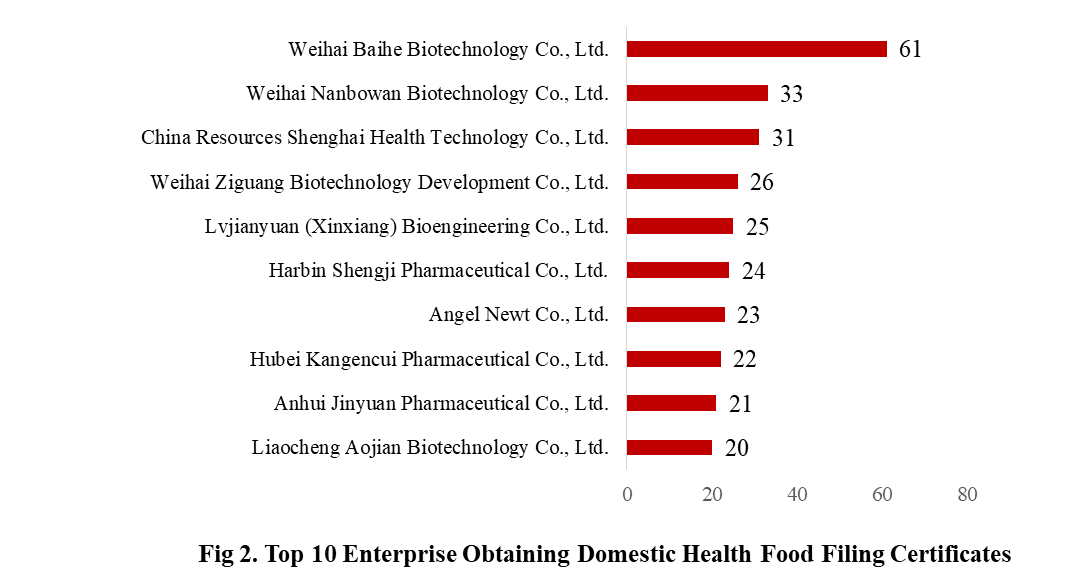
There are 369 domestic health food manufacturers obtained health food filing certificates. Weihai Baihe Biotechnology Co., Ltd. has the largest number of approvals, and obtained a total of 61 filing products, followed by Weihai Nanbowan Biotechnology Co., Ltd. and China Resources Shenghai Health Technology Co., Ltd., with the number of 33 and 31, respectively.
4. The Filing Status of Health Food in Different Dosage Forms
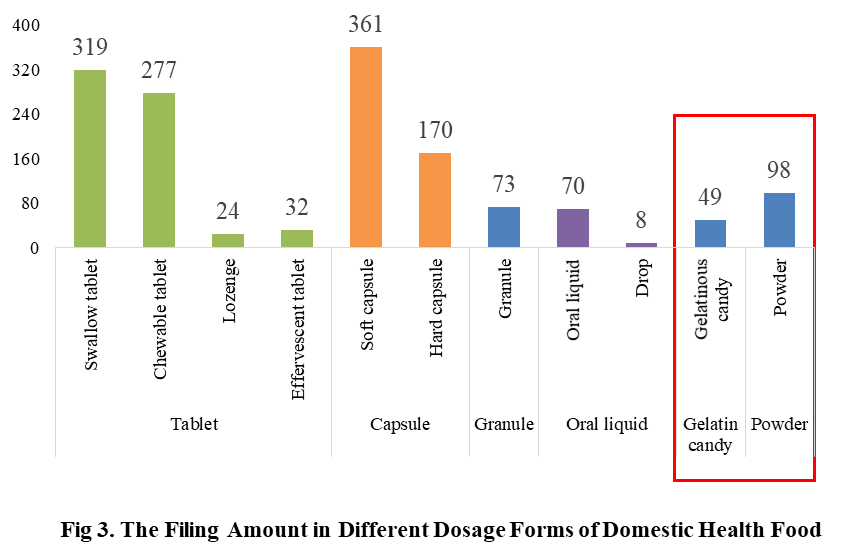
At present, the permitted dosage forms for filing include tablet, capsule (hard/soft), oral liquid, granule, powder and gelatinous candy.
The main dosage form for domestic health food filing is tablet with the number of 652, accounting for 44% of the total. The capsule include soft capsule and hard capsule, and the number of filings is 531. The quantity of soft capsule products is far higher than the number of hard capsule products, which are 361 and 170 products respectively. In addition, the quantity of oral liquid (including drop) and granule products are 78 and 73 respectively. Among oral liquid products, there are only 8 drop products.
Powder and gelatinous candy (gummy) are the new dosage forms available for filing since June 1, 2021, 98 and 49 products respectively in these 2 dosage forms have got the filing certificates in 2022.
5. The Filing Status of Health Food in Different Functional Ingredients
Among the filed domestic health foods in 2022 (as of July 5), there are 910 nutrition supplements, and 571 functional health food with broken spore ganoderma lucidum powder, melatonin, fish oil, coenzyme Q10 or spirulina as single raw material. Among the nutrition supplements, the products with the function of supplementing multi-vitamins & minerals are the most (348 products are filed), followed by products supplementing a single nutrient and supplementing two nutrients, the number are 336 and 226 respectively.
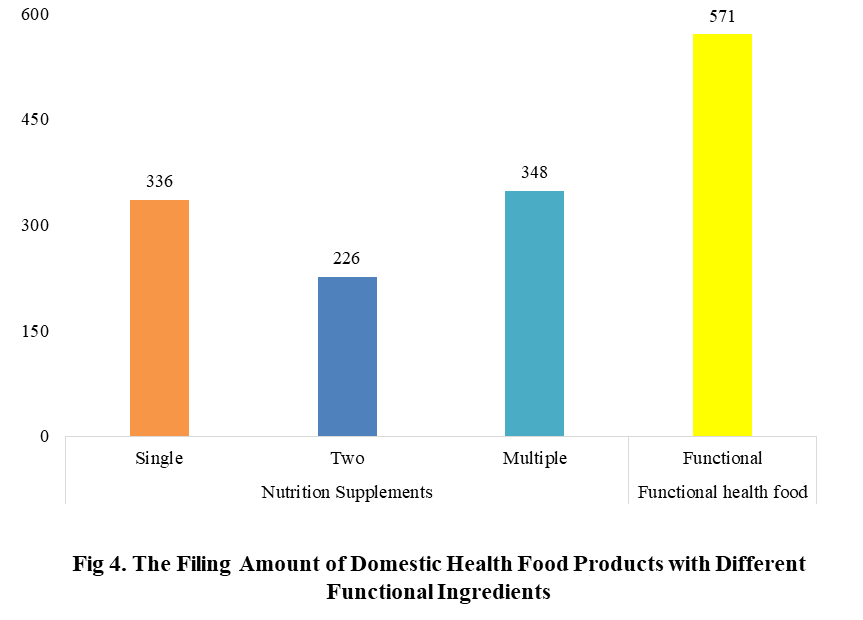
Among the domestic filed products, the most popular nutrition supplements are Vitamin C supplements, Calcium & Vitamin D supplements and Multi-vitamins & minerals supplements, the number of them are 144, 93 and 226 respectively.
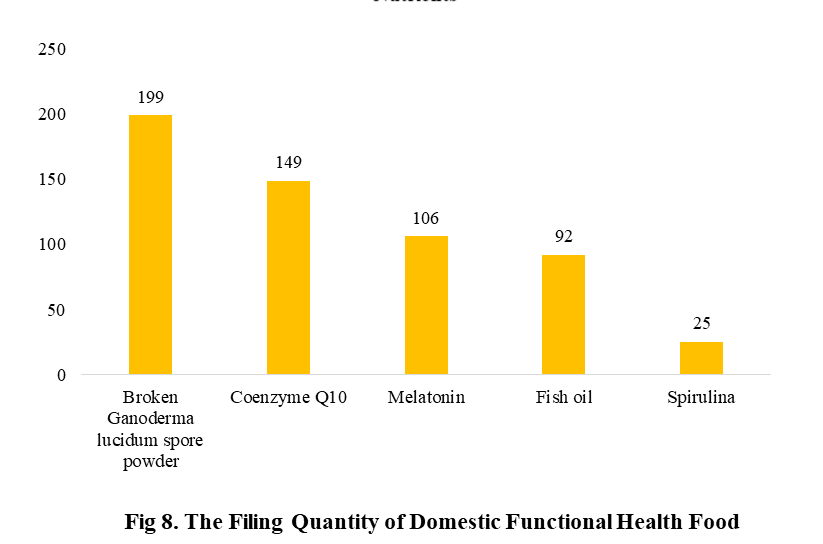
The functional health food with broken spore ganoderma lucidum powder, coenzyme Q10,melatonin, fish oil or spirulina as the single raw material have been transferred to filing supervision on June 1, 2021. Their filing numbers are 199, 149, 106, 92 and 25 in 2022 (as of July 5).
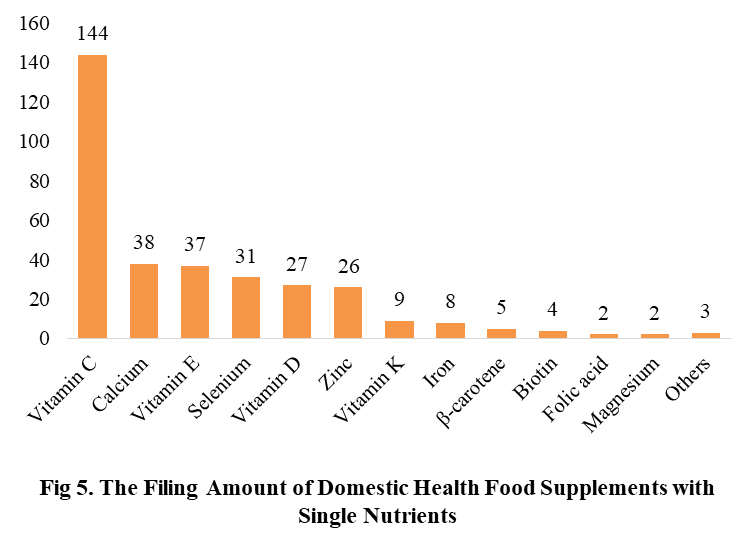
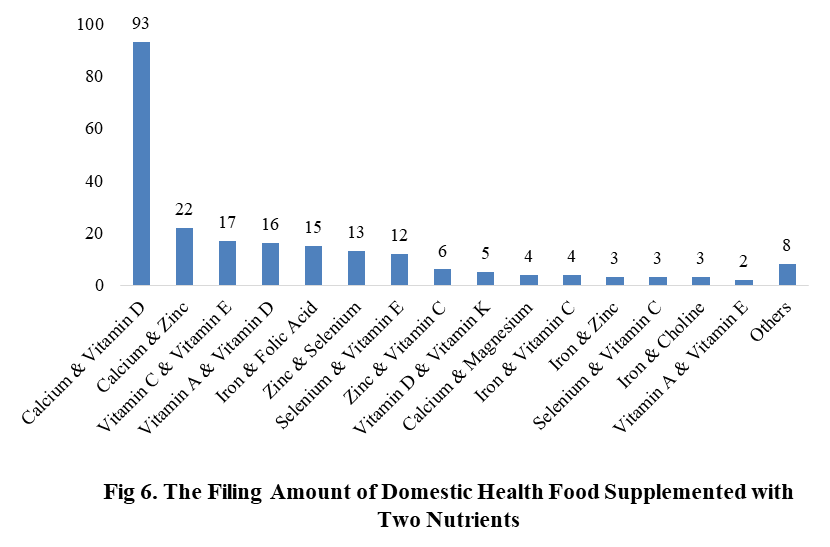
Note: Nutrition supplements with less than 2 products are classified as “Other”.
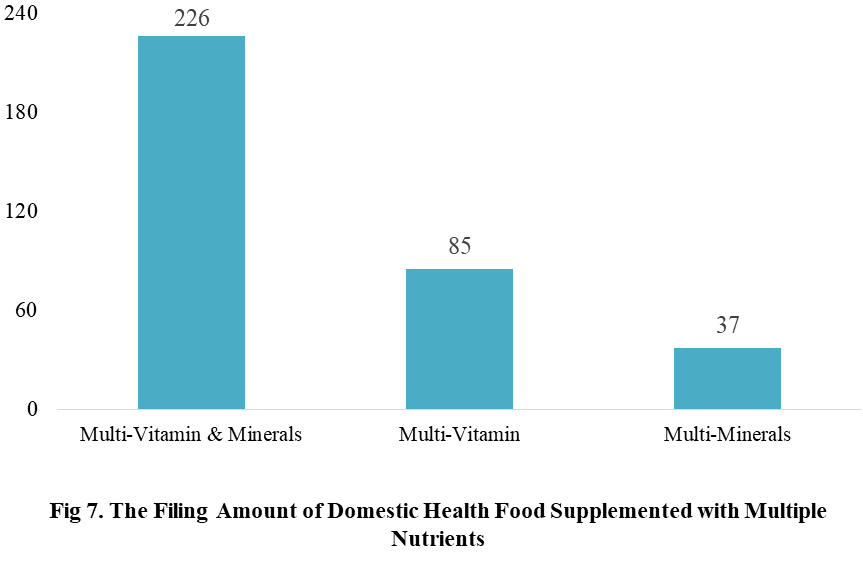
CIRS Comments
After the health food withbroken spore ganoderma lucidum powder, melatonin, fish oil, coenzyme Q10 or spirulina as the single raw material is transferred from registration to filing management, the application period is effectively shortened, the cost of enterprises is reduced, and related products can be launched more quickly. In 2022 (as of July 5), 571 functional health food have obtained filing certificates (such product filing is currently only applicable to domestic products), accounting for 38.5% of the total. We believe that in the future, with the continuous expansion of the catalogue of health food raw materials and the continuous improvement of the dual-track system of health food, there will be more health food categories into the public view, and inject vitality into the Chinese market.
Note:
I. The data in this article is from the Special Food Information Query Platform.
II. There may be some omissions in the data of the Special Food Information Query Platform, thus the data in this article is for reference only, and please refer to the information published by the government.
If you have any needs or questions, please contact us at service@hfoushi.com.
Reference
Analysis on China Health Food (Dietary Supplement) Registration Products in the First Half of 2022

