According to the
New Food Safety Law of the People’s Republic of China
, China Food Drug Administration (CFDA) issued the
Administrative Measures on Health Food Function Directory and Food Raw Material Directory
(draft version for public comments) on July 28th 2015, in an attempt to regulate the management of function directory and raw material directory for health food. The public can present opinions and advices for the measure before August 28th this year. With the publishing of this measure, related manufacturer, research institution and other organization are permitted to develop new functions and raw health food materials.
1. The administrative measure of function directory for health food.
1.1 What is function directory?
Function directory refers to the list of healthy function information that allowed to be claimed in the label of health food, which has clear evaluation methods and criteria after a comprehensive evaluation and verification.
1.2 How to use the function directory?
The claim of health food function in the label should be strictly in accordance with the description in the function directory. Behaviors, such as “increase or decrease the words”, or “make random combination”, are forbidden.
1.3 The procedures of the function directory application
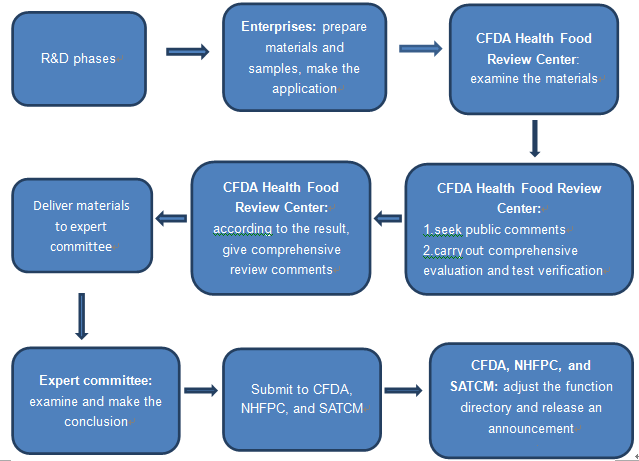
1.4 The required materials of function directory application and related requirement
|
No. |
Required application materials | Requirements | Exclusion items |
|
1 |
Name of the health food function |
For the purpose of regulating body functions, improving the body’s health status or reducing the disease risk |
1. Expression or implied expression of disease prevention, treatment, diagnosis, or easily confused.
|
|
2 |
Naming basis of the function |
Have sufficient scientific evidences, and can be understood correctly |
|
|
3 |
R&D materials of the function |
1. With clear suitable and non-suitable crowds;
|
|
|
4 |
Evaluation methods, judgment criteria, and function test report of the corresponding sample |
Have scientific evaluation methods and judgement criteria |
|
|
5 |
The function-related materials at domestic and foreign |
|
|
|
6 |
Scientific literature and other related materials |
|
|
|
7 |
For function adjusting application : adjustment reason, the basis and related materials are additional needed. |
2. The administrative measure of raw material directory for health food
2.1 What is raw material directory?
Raw material directory refers to the information list of materials that allowed to be used in health food, which has been evaluated on safety and function. Contents of this directory include: the name of the raw material, compatibility, dosage, allowed function declare, quality standard, functional component, detection methods and related instructions.
2.2 How to use the raw material directory?
2.2.1 If the health food is produced strictly according to the information in raw material directory, it can carry out health food filing.
2.2.2 If the product is produced by materials listed in raw material directory, but with the processes such as extraction and purification, it should make the health food registration.
2.2.3 Raw materials for supplying the nutrients like vitamins, minerals in the raw material directory can make a combination, other raw materials cannot be combined.
2.2.4 For more than two kinds of raw material combination, if they have synergistic effect or promotion effect, and comply with the requirements of the administrative measures, and there are sufficient scientific evidences and application history for the combination, it can also be applied to getting into the raw material directory.
2.3 The procedures of the raw material directory application
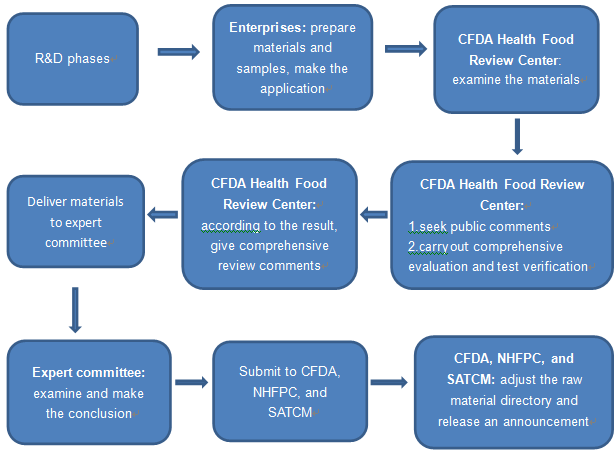
2.4 The required materials of raw material directory application and related requirements.
| No | Required application materials | Requirements | Exclusion items |
| 1 | Name of raw material, including the standard Chinese name, Latin name, family and genus species |
1. No health food consumption record
2. After food safety risk assessment, the raw material may have uncertain factors, or some potential hazards to human health 3. Forbidden to consumption or do not meet the relevant national wildlife protection laws and regulations 4. Raw materials which are unable to standardization management or industrial production |
|
| 2 | Source and specifications (for the raw material from animal or plant: the usage part, traditional Chinese medicine literature are also needed) | ||
| 3 | Daily dosage range and the corresponding function effect |
1. Have a clear dosage range and the corresponding function are in compliance with function directory.
2.When consumed according to the prescribed dosage and method, it is harmless and safety to the suitable population |
|
| 4 | Records of adverse food safety problems and related reports | ||
| 5 | Main process requirements | Have stable and controllable quality and technical requirements | |
| 6 | Quality standards | ||
| 7 | Range of the functional component or sign composition, and the detection methods | Have scientific, stable and reliable functional component or sign composition and detection methods. | |
| 8 | Materials about the suitable and non-suitable crowds | ||
| 9 | Limited use conditions and precautions | ||
| 10 | The usage of raw materials in domestic and foreign, including the usage in the health food has been approved in China |
1.Have wide usage history in domestic and foreign
2.Have the scientific evidence that the material is in compliance with safety and effectiveness requirements |
|
| 11 | Other scientific literatures | ||
| 12 | Other related materials | ||
| 13 | For raw material directory adjusting application : adjustment reason and related materials are additional needed. |

