On January 19, 2023, China National Center for Food Safety Risk Assessment (CFSA) released National Food Safety Standard - Standard for the Use of Food Nutrition Enhancers (Draft) for public comment. Significant changes have been made and we have summarized them as follows:
1. Relevant regulations from official announcements are included
A total of 20 regulations related to nutrition enhancers from official announcements issued by National Health Committee (NHC) from 2012 to 2022 have been added to the standard.
2. Revisions to texts
a) Clarifying the category and definition of general food fortification and voluntary food fortification:
- General food fortification: the addition of one or more micronutrient(s) to specific generally consumed food, usually organized and implemented by related government departments;
- Voluntary food fortification: the addition of one or more micronutrient(s) and/or other vitamins or minerals to foods (except for the food with general food fortification) at the discretion of the manufacturer for specific purposes.
b) Deleting the definition of food for special dietary uses;
c) Revising the requirements for the use of nutrition enhancers, requirements for the selection of food categories that can be fortified, and regulations on the use of nutrition enhancers.
3. Revisions to annexes
3.1 Annex A & Annex B: Regulations on the use of nutrition enhancers in general foods and voluntary foods
Notable changes in this revision are as follows:
a) The existing Annex A “Regulations for the use of nutrition enhancers in food” is adjusted to Annex A “Regulations for the use of nutrition enhancers in general food” and Annex B “Regulations for the use of nutrition enhancers in voluntary food”
General foods listed in Annex A
|
Cat No |
Food Category |
|
01.01 01.02 |
Pasteurized milk, sterilized milk and modified milk Fermented milk and flavored fermented milk |
|
02.01.01.01 |
Vegetable oils |
|
06.02.01 |
Rice |
|
06.03.01 |
Wheat flour |
|
12.04 |
Soybean sauce |
Annex A.1 specifies the types and usage requirements of nutrients that must be fortified in categories of general food fortification, while Annex A.2 specifies those that can be fortified selectively. The general food fortification shall meet the requirements of Annex A, which requires mandatory fortification of all the nutrients listed in Annex A.1, based on which the nutrients listed in Annex A.2 can be fortified selectively;
Annex B specifies the types and usage requirements of nutrients in categories of general food fortification.
b) The Draft revises the layout for Annex A and B from a vertical layout to a horizontal one, making it clearer and more concise.
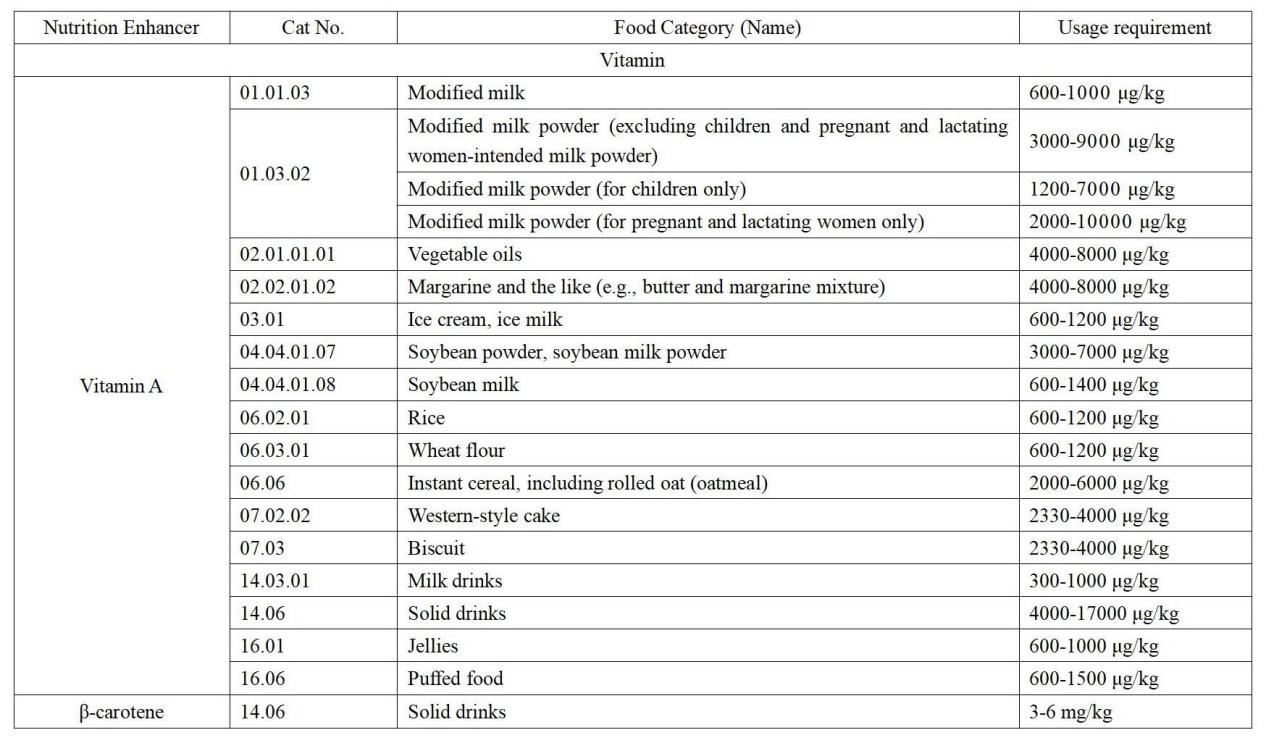
Figure 1. Display form in GB 14880-2012 (vertical)
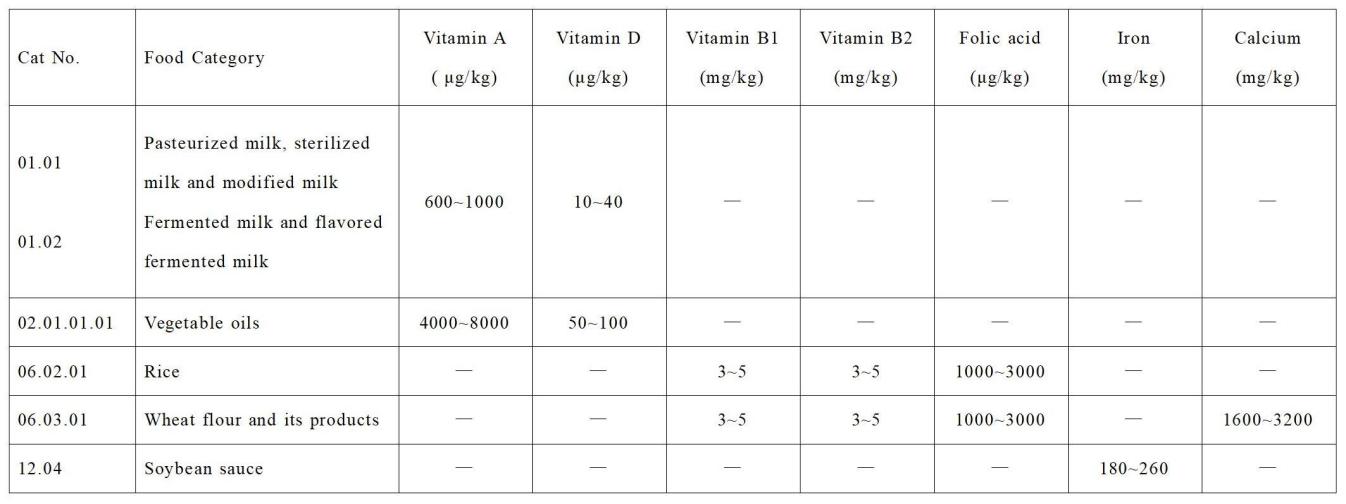
Figure 2. Display form in GB 14880 (draft) (horizontal)
3.2 Annex C & D: the list of compound sources of nutrition enhancers permitted for use in Annex C and the nutrition enhancers and their compound sources permitted for use in foods for special dietary uses in Annex D (excerpts)
- Removing the nutrition enhancers such as ferrous fumarate, zinc carbonate, bone powder (superior fine and fresh bone powder), γ- linolenic acid;
- Including isomerized lactose;
- Including ferrous lactate in Annex D;
- Adding the remark "except for infant formula and infant formula for special medical uses" to ferric sodium ethylene diamine tetraacetate in Annex D;
- Casein calcium peptide is revised as Casein phosphopeptide
- Phytonadione is revised as Vitamin K;
- Ascorbyl palmitate is revised as L-ascorbic acid-6-palmitate (ascorbyl palmitate);
- 6S-5-methyltetrahydrofolate calcium is revised as (6S)-5-methyltetrahydrofolic acid;
- Arachidonic acid (AA or ARA) is revised as eicosatetraenoic acid (arachidonic acid) (AA or ARA); and
- Fructo-oligosaccharide (from witloof) is revised as fructo-oligosaccharide.
3.3 Annex E: food category (name) description
- Adding several food categories, e.g., cream powder, modified cream powder, other grain and its products;
- Deleting several food categories, e.g., water-based flavored drinks and non-carbonated drinks;
- Revising the names of several food categories, e.g., tea drinks and coffee drinks;
- Revising certain food categories, e.g., the infant formula for special medical purposes is moved from formula for infants and young children category to the foods for special medical purposes category.
CIRS Comments
GB 14880-2012 has been implemented for ten years, during which several announcements were issued to improve the regulations on the use of food nutrition enhancers. Undoubtedly, the release of this draft will contribute to the improvement of the whole industry. The deadline for comments is February 28, 2023.
If you need any assistance or have any questions, please get in touch with us via service@hfoushi.com.

