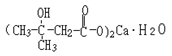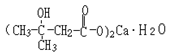With the vigorous development of the nutrition and health industry in China and further standardize the licensing examination work, more and more new food raw material has been widely used after approval. The use of some approved raw materials is restricted. If enterprises want to expand the use scope, they also need to do the application in order to achieve the purpose of expanding the market.
In order to help enterprises obtain relevant information quickly, CIRS Group summarized a list of new food raw materials that have been "expanded in use" since 2008.
1. Food raw materials that are "expanded in use" by Notice
Sodium Hyaluronate
|
Content of original Notice (2008) |
Content of new Notice (2021) |
|
|
Latin/English name |
Sodium Hyaluronate |
Sodium Hyaluronate |
|
Basic information |
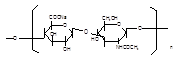
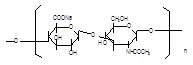
Source: Streptococcus equi subsp. Zooepidemicus Structural formula:
Molecular formula: (C14H20NNaO11)n, n=200-10000 Molecular weight: 8.02×104–4.01×106 |
Source: Streptococcus equi subsp. zooepidemicus Structural formula:
Molecular formula: (C14H20NNaO11)n, n=200-10000 Molecular weight 8.02×104–4.01×106 |
|
Production process |
Using glucose, yeast powder, peptone as the culture medium, fermented from Streptococus equi subsp.zooepidemicus. |
Using glucose, yeast powder, peptone as the culture medium, fermented from Streptococus equi subsp.zooepidemicus. |
|
Application scope |
Health food raw materials |
1) Milk and dairy products (0.2 g/kg); 2) Beverages (Liquid beverage: ≤50 mL package 2.0 g/kg, 51-500 mL package 0.20 g/kg; Solid beverage is converted according to the volume of liquid after quenching and adjustment); 3) Alcohol (1.0 g/kg) 4) Cocoa products, chocolate and its product (including imitations and chocolate substitutes), candy (3.0 g/kg); 5) Frozen drinks (2.0 g/kg) |
|
Recommended intake |
≤200 mg/d |
≤200 mg/d |
|
Quality requirement |
Property: White granule or powder |
Property: White granule or powder |
|
Sodium hyaluronate content ≥87.0% |
Sodium hyaluronate content (g/100g): ≥87.0 |
|
|
Moisture ≤10.0% |
Moisture (g/100g): ≤10.0 |
|
|
pH: 6.0-8.0 |
pH: 6.0-8.0 |
|
|
Ash ≤13.0% |
Ash (g/100g): ≤13.0 |
|
|
Other information |
/ |
1. Unsuitable group: infants, pregnant women and lactating women. Labels and instructions should indicate the unsuitable group and the recommended consumption should be≤200 mg/d; 2. Food safety indicators must meet the following requirement: Pb ≤0.5 mg/kg As≤0.3 mg/kg |
|
Notice date |
2008-05-30 (2008 Notice No. 12) |
2021-01-07 ( 2020 Notice No, 9 ) |
Calcium β- hydroxy -β- methyl butyrate
|
Content of original Notice (2011) |
Content of new Notice (2017) |
|
|
Latin/English name |
Calcium β- hydroxy -β- methyl butyrate (CaHMB) |
Calcium β-hydroxy -β-methyl butyrate (CaHMB) |
|
Basic information |
Structural formula:
Molecular formula: C10H18O6Ca•H2O Molecular weight: 292 |
Structural formula:
Molecular formula: C10H18O6Ca•H2O Molecular weight: 292 |
|
Production process |
With sodium hypochlorite, diacetone alcohol, hydrochloric acid, ethyl acetate, ethanol, calcium hydroxide as the main raw materials, produced by oxidation synthesis, acidification, extraction, neutralization reaction, centrifugation, drying and other steps. |
With sodium hypochlorite, diacetone alcohol, hydrochloric acid, ethyl acetate, ethanol, calcium hydroxide as the main raw materials, produced by oxidation synthesis, acidification, extraction, neutralization reaction, centrifugation, drying and other steps. |
|
Recommended intake |
≤3g/d |
≤3g/d |
|
Application scope |
Sports nutrition foods, foods for special medical purpose |
Beverages, milk and dairy products, cocoa products, chocolate and its products, candy, baked foods, sports nutrition foods, foods for special medical purpose |
|
Quality requirement |
Property: White powder β- hydroxy -β- methyl butyrate: 77-82% Ca: 12-16% Moisture: 5-7.5% |
Property: White powder β- hydroxy -β- methyl butyrate:(g/100g): 77-82 Ca (g/100g): 12-16 Moisture (g/100g): 5-7.5 |
|
Other information |
Unsuitable group: Infants, children, pregnant women and lactating women. Labels and instructions should indicate the unsuitable group and the Recommended intake. |
1. Unsuitable group: Infants, children, pregnant women and lactating women. Labels and instructions should indicate the unsuitable group and the Recommended intake. 2. Hygiene and safety indicators shall conform to the relevant national standards |
|
Notice date |
2011-01-21 (2011 Notice No.1) |
2017-06-08 (2017Notice No.7) |
In addition, three new food raw materials (plant stanol ester, cordyceps militaris and sucrose polyester) were also updated in the second Notice. Except for the change or adjustment of source, recommended intake, quality specification requirements and production process requirements, the scope of use is not limited (that is, there is no restriction on the scope of use) in the second Notice.
Compared with the original Notice, the main changes of these three food raw material are as follows:
1) Plant stanol ester: tarot oil was added as the raw material,and there is no restriction on the scope of use;
2) Cordyeps militaris: the requirements of recommended intake, quality index and application scope are no longer limited;
3) Sucrose Ployesters: change the recommended intake to≤10.6g/d,the requirements of application scope are no longer limited;.
2. Food raw materials that are "expanded in use" by Termination
Isodon lophanthoides(Buchanan-Hamilton ex D.Don)H.Hara var. gerardianus(Bentham)H.Hara
|
Content of original Notice (2013) |
Termination (2021) |
||
|
Latin/English name |
Isodon lophanthoides(Buchanan-Hamilton ex D.Don)H.Hara var. gerardianus(Bentham)H.Hara |
Product name |
Rabdosia serra (Maxim.) Hara (It was later renamed as “Isodon lophanthoides(Buchanan-Hamilton ex D.Don)H.Hara var. gerardianus(Bentham)H.Hara”. |
|
Basic information |
Source:cultivated Isodon lophanthoides(Buchanan-Hamilton ex D.Don)H.Hara var. gerardianus(Bentham)H.Hara Species: lamiaceae, Isodon |
Review comment |
The product name shall be Isodon lophanthoides(Buchanan-Hamilton ex D.Don)H.Hara var. gerardianus(Bentham)H.Hara. It has been approved as a new food raw material for tea drinks, and this application is extended to substitute tea. Based on the historical data and safety assessment data provided, it was agreed toexpand to substitute tea. It is suggested to terminate the examination, and the food safety indicators shall be implemented according to the following contents: Lead (count as Pb) ≤2 mg/kg; Total arsenic (count as As) ≤0.5 mg/kg; BHC ≤0.02 mg/kg; DDT≤0.02 mg/kg. |
|
Recommended intake |
≤ 8g/d |
||
|
Other information |
1. Application scope: tea beverages. 2. Unsuitable group: Infants, young children and pregnant women. Labels and instructions should indicate the unsuitable group and the Recommended intake. 3. Hygiene and safety indicators shall conform to the relevant national standards. |
||
|
Notice date |
2013-11-26 (2013Notice No. 10) |
Link |
Included in the list of Termination in 2021 |
Penthorum chinense Pursh.
|
Content of original Notice (2013) |
Termination (2021) |
||
|
Latin/English name |
Penthorum chinense Pursh. |
Product name |
Luzhou Gulin Penthorum chinense Pursh (It was later renamed as “I Penthorum chinense Pursh”. |
|
Basic information |
Species: Saxifragaceae, penthorum Edible parts: Stems and leaves |
Review comment |
It has been approved as a new food raw material as brew drink, and this application is extended to beverages. Based on the historical data and safety assessment data provided, it was agreed toexpand to beverages. It is suggested to terminate the examination. Except for the eating method, others shall follow the 2020 Notice No. 4. |
|
Production process |
Produced by selecting, cleaning, rinsing, cutting and drying the stems and leaves of cultivated penthorum chinense Pursh. |
||
|
Recommended intake |
≤ 8g/d |
||
|
Other information |
1.Unsuitable group: Infants, young children, Pregnant and lactating women. Labels and instructions should indicate the unsuitable group and the Recommended intake. 2. Eating method: brew drink 3. Hygiene and safety indicators shall conform to the relevant national standards. |
||
|
Notice date |
2020-06-02 (2020 Notice No. 4) |
Link |
Included in the list of Termination in 2022 |
The research results of each new food raw material are gradually accumulated with the progress and development of science. The safety, dosage, or application scope of a food raw material may undergo updating or change. For new raw materials that do not meet the requirements of the original notice or need to be expanded in use scope, a new application shall be submitted. It can be used only after it is approved by NHC. The final approval results may be published by a new notice, or included in the termination list.
(Note: The data in this paper are for reference only.)
If you have any needs or questions, please contact us atservice@hfoushi.com.