Background
The U.S. Food and Drug Administration (FDA) is a federal regulatory agency in the Department of Health and Human Services (DHHS) of the United States. As a scientific regulatory agency, FDA is responsible for ensuring the safety of food (including food additives), food contact materials, medical devices, radiological products, and drugs produced or imported into the United States. These products must be registered or certified by the FDA before they can be sold in the US market, representing the highest honor and assurance sought by manufacturers.
Facilities engaged in manufacturing, processing, packing, or holding food for consumption (including food additive, health food, etc.) are required to submit an registration to FDA according to the Federal Food, Drug and Cosmetic Act (FD&C Act). In addition, it is required to renew the registration of your facility with FDA every other year during the period beginning on October 1 and ending on December 31 of each even-numbered year, and ensure to allow FDA facility inspections as permitted by the FD&C Act.
Examples of the types of food that have to register with FDA
|
INCLUDED Foods |
EXCLUDED Foods |
|
Dietary supplements and dietary ingredients |
Food contact substances |
|
Infant formula |
Pesticides |
|
Beverages (including alcoholic beverages andbottled water) |
|
|
Fruits and vegetables |
|
|
Fish and seafood |
|
|
Dairy products and shell eggs |
|
|
Raw agricultural commodities for use as food or components of food |
|
|
Canned and frozen foods |
|
|
Bakery goods, snack food, and candy(including chewing gum) |
|
|
Live food animals |
|
|
Food for animals (e.g., pet food, pet treats andchews, animal feed) |
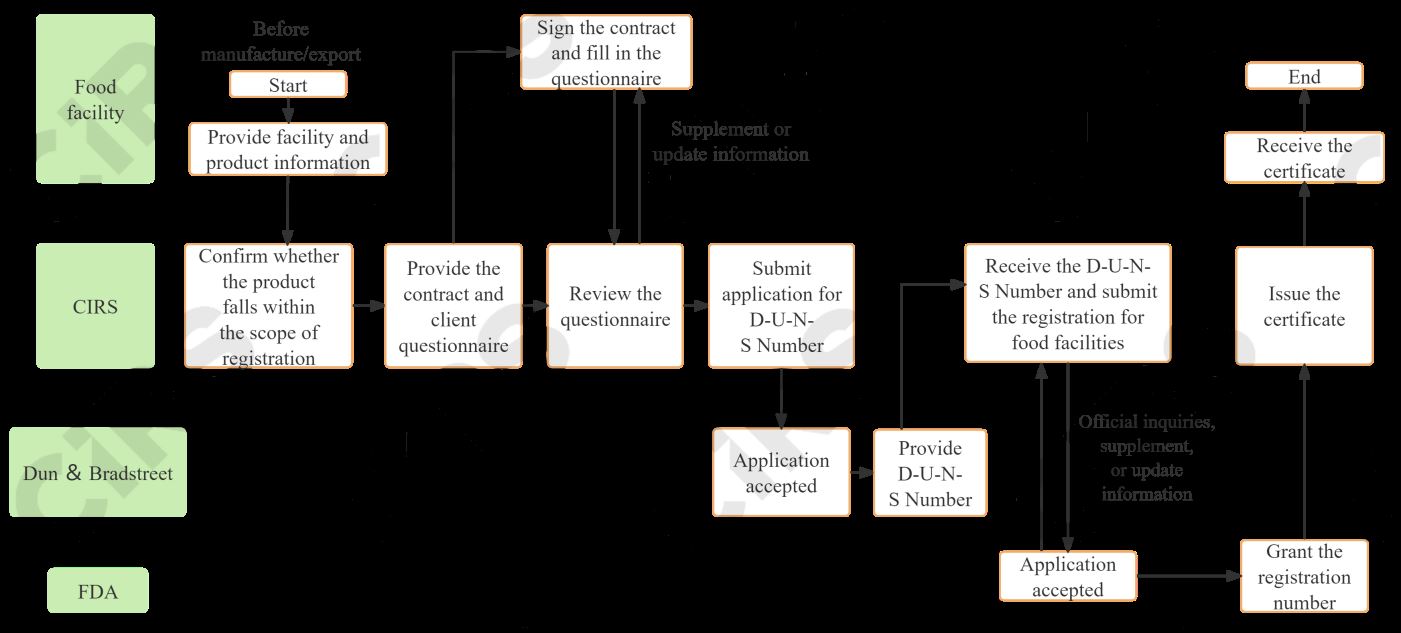
Registration procedures
A foreign facility must appoint a domestic agent within the United States when applying for registration for food facilities. Additionally, a D-U-N-S Number is required, which serves as a unique facility identifier for the company.
Information required for registration
- Facility name, full address, phone number;
- D-U-N-S Number;
- The preferred mailing address, if different from that of the facility;
- Parent company name, address, and phone number (if applicable);
- Email address for the contact person of the facility or, in case of a foreign facility, the Name, address, phone number, and email address of the U.S. Agent for the facility;
- An emergency contact phone number and email address;
- Name, full address and phone number of the owner, operator, or agent in charge. In addition, the email address of the owner, operator, or agent in charge is required, unless FDA has granted a waiver under 21 CFR 1.245;
- All trade names the facility uses;
- Applicable food product categories, as listed on the registration form;
- The type of activity conducted at the facility for each food product category identified;
- Assurance that FDA will be permitted to inspect the facility at the times and in the manner permitted by the FD&C Act; and
- Certification that the information submitted is true and accurate and that the person submitting it is authorized to do so.
FAQs
Q1: Is it necessary to appoint a U.S. agent when an overseas company applies for the food facilities registration?
A1: Yes, all overseas companies must appoint an U.S. agent to facilitate communication with the FDA when registering.
Q2: What it costs?
A2: There is no fee for registration. The comoany only needs to pay the service fees to the agent.
Q3: How long does the registration take?
A3: The timeframe depends on the completeness of the materials required. Generally, it takes between 2 weeks to 1 month.
Q4: Is there a certificate issued after I registered my food facility?
A4: After the registration is approved, the U.S. FDA does not issue any certificates. However, the company will receive an FDA Facility Registration, which will also be used for Prior Notice submissions. We will provide you with an official certificate to confirm that your food facility has completed the FDA registration.
Q5: Does the registration have an expiration period?
A5: A food facility is required to submit an initial registration to FDA only once. Section 415(a)(3)of the FD&C Act, as amended by section 102 of FSMA, requires your facility to renew its registration with FDA every other year during the period beginning on October 1 and ending on December 31 of each even-numbered year.
Q6: When do I submit the Prior Notice for exporting food ? What it costs?
A6: It should be submitted no earlier than 15 days before the arrival date (if submitted via PNSI) or no earlier than 30 days before the arrival date (if submitted via ABI/ACS). It can be submitted either by the exporter or the importer, and the FDA does not charge any fees for this. If you need assistance from an agent, only a small service fee is required.
Our services
- US FDA Certification consultation and training
- US FDA Food Facility Registration
- US D-U-N-S Number application
- US Prior Notice
- US Food Additive Petitions (FAP) application
- US FDA GRAS Certification application
- US FDA Food Contact Materials Certification
Our strength
CIRS Group boasts a wholly-owned subsidiary in the United States, specializing in EPA and FDA related certifications. With a professional team experienced in testing and certification, we offer short turnaround times, competitive pricing, and tailored one-on-one consulting services. Feel free to reach out or visit us to learn more about our comprehensive technical capabilities!