From April 7 to April 14, 2024, the National Health Commission (NHC) Government Services Platform issued notifications for the approval and review of “three new foods” (new food raw materials, new food additives, and new food-related products). This includes delivering an extension notice for 1 new food raw material and issuing a non-approval decision on 1 new food-related product. Details are as follows:
1. Extension notice (requiring supplementary materials following the technical review by China National Center for Food Safety Risk Assessment, CFSA)
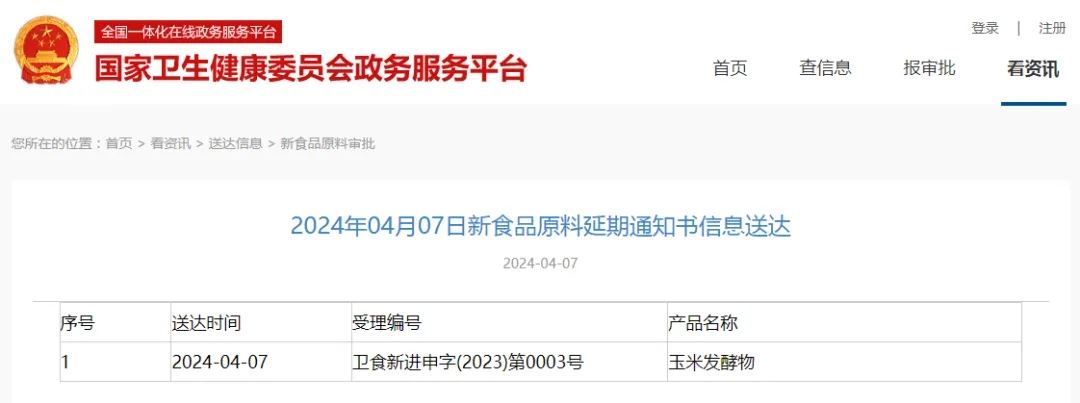
|
No. |
Date of delivery |
Acceptance number |
Product name |
|
1 |
2024.4.7 |
卫食新进申字(2023)第0003号 |
Corn fermentation |
2. Non-approval decision (based on the recommendation from NHC not to grant approval)
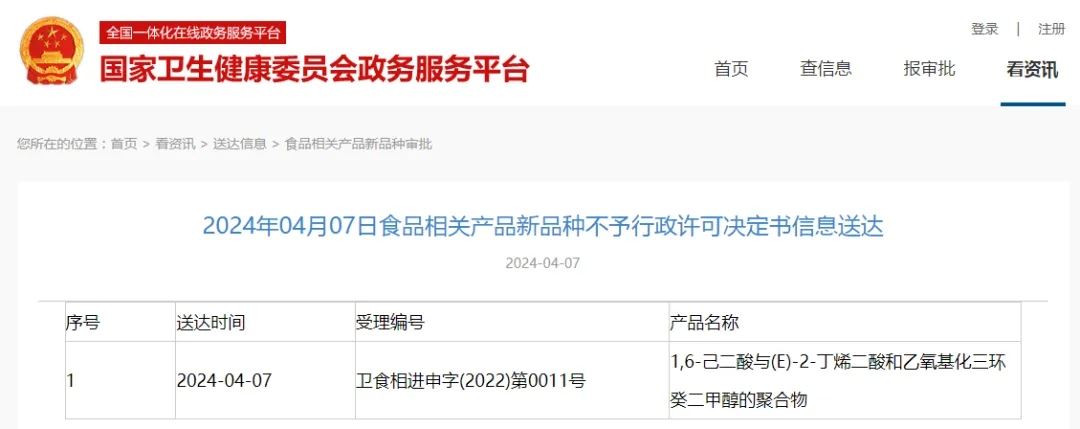
|
No. |
Date of issuance |
Acceptance number |
Product name |
|
1 |
2024.4.7 |
卫食新进申字(2022)第0011号 |
hexanedioic acid, polymer with (e)-2-butenedioic acid and octahydro-4,7-methano |
If you need any assistance or have any questions, please get in touch with us via service@hfoushi.com.
Further information
National Health Commission Government Services Platform

